RACEMIC MODIFICATION (RACEMATE || RACEMIC MIXTURE): SEPARATION OF ENANTIOMERS-RESOLUTION
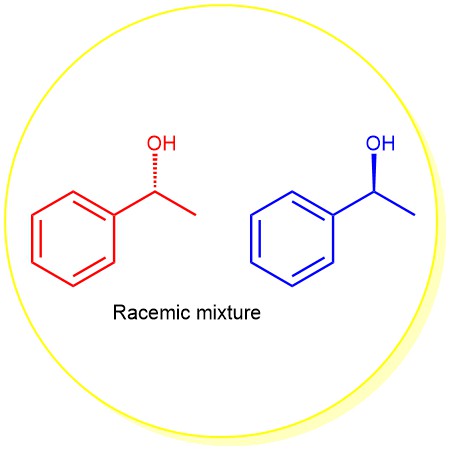
An equimolar mixture of two enantiomers is called a racemic modification (or racemate or racemic mixture). When enantiomers are mixed together in equal proportion, the rotation caused by a molecule of one isomer is exactly canceled by an equal and opposite rotation caused by a molecule of its enantiomer. As a result, the racemic modifications …
RACEMIC MODIFICATION (RACEMATE || RACEMIC MIXTURE): SEPARATION OF ENANTIOMERS-RESOLUTION Read More »