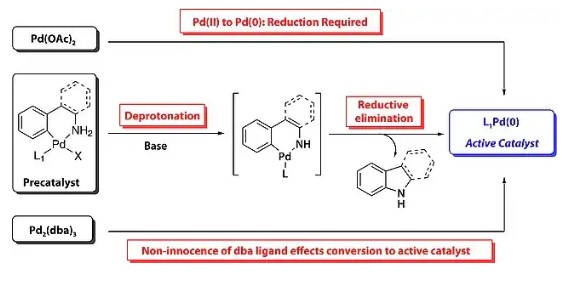
Aryl amines and related heterocycles are essential components in natural products, pharmaceuticals, agrochemicals, and various other compounds. This significance has driven chemists to seek advanced methods for forming carbon-nitrogen (C-N) bonds efficiently.
Historically, the formation of C(sp2)-N bonds has been achieved using nucleophilic aromatic substitution (SNAr) or Goldberg and Ullmann reactions. In 1983, Nigita and colleagues pioneered the use of palladium complexes as catalysts for creating C(sp2)-N bonds. They discovered that palladium complexes containing P(o-tol)3 can catalyze the synthesis of aryl amines from aryl bromides and amino stannanes.
Stephen L. Buchwald and John F. Hartwig introduced an enhanced protocol in 1994 for coupling aryl bromides and amino stannanes. Subsequently, in 1995 they proposed a tin-free palladium-catalyzed method for coupling aryl bromides and amines.
Today, the Buchwald-Hartwig coupling reaction refers to the direct palladium–catalyzed formation of C-N bonds between aryl halides or triflates and amines in the presence of a stoichiometric quantity of a base. In this process, the aryl halides (or pseudohalides) act as electrophiles, while the nucleophiles encompass various amines (both aliphatic and aromatic), amides, imides, sulfonamides, urea, and more.

The choice of ligands for the palladium catalyst plays a crucial role in the success of the Buchwald-Hartwig coupling reaction, influencing the efficiency of C-N bond formation. Buchwald Catalysts & Ligands (click)
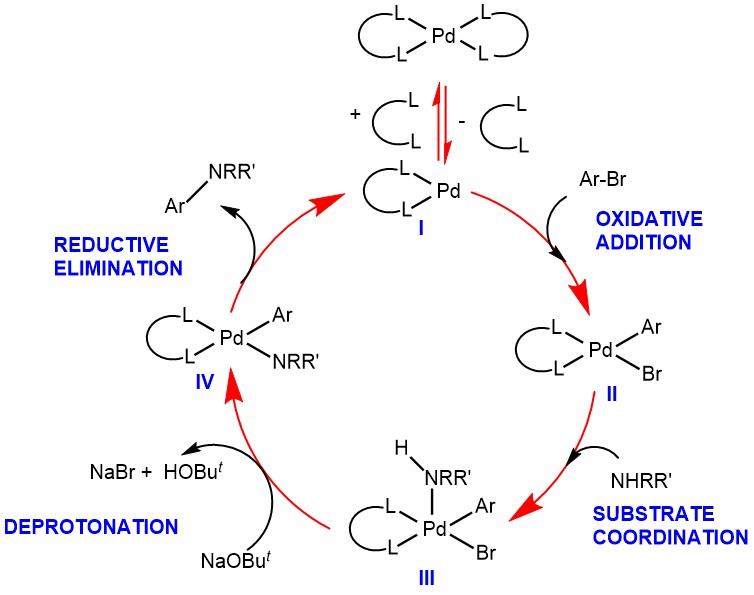
MECHANISM: When BINAP is used as the bident ligand, oxidative insertion of Pd(0) species (I) into the aryl halide substrate affords the Pd(II)-complex (II). Coordination of the amine significantly increases its acidity, allowing its deprotonation by the bases such as LiHMDS, NaOBut forming Palladium amide-complex (IV), which undergoes reductive elimination to give the aryl amine (C-N bond) bond and the regeneration of the Pd(0) catalyst (I).
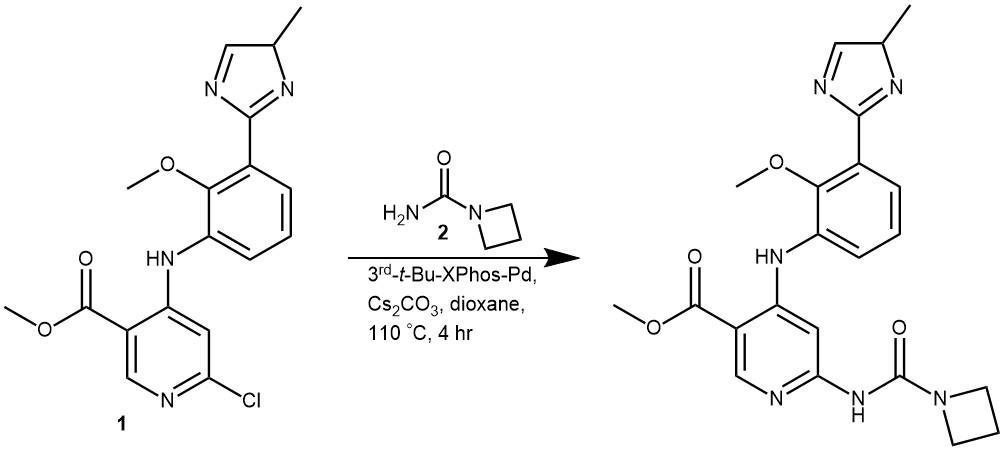
EXAMPLE 1: To a solution of (1) (100 mg, 0.27 mmol, 1.0 eq) in dioxane (5 mL) were added (2) (81 mg, 0.81 mmol, 3.0 eq), Cs2CO3 (175 mg, 0.54 mmol, 2.0 eq), and 3rd–t-Bu-Xphos (24 mg, 0.027 mmol, 0.1 eq). The reaction was stirred for 4 hours at 110 °C under nitrogen. The mixture was filtered and the filtrate was concentrated. The crude product was purified by prep-TLC (DCM/MeOH = 15/1) to give the desired product (43.7 mg, 37.3% yield) as a yellow solid. (REF: World Intellectual Property Organization WO 2020/086616 AI)
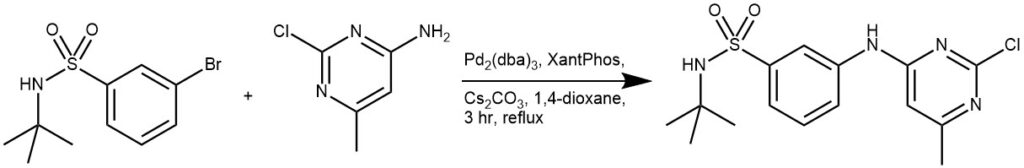
EXAMPLE 2: A mixture of 2-chloro-5-methyl-pyrimidin-4-ylamine (0.4 g, 2.8 mmol), 3-bromo-t-butyl-benzenesulfonamide (1.0 g, 3.4 mmol), Pd2(dba)3 (0.17 g, 0.19 mmol), Xantphos (0.2 g, 3.5 mmol) and cesium caibonate (2.0 g, 6.1 mmol) was suspended in dioxane (25 mL) and heated at reflux under the argon atmosphere for 3 h. The reaction mixture was cooled to room temperature and diluted with DCM (30 mL). The mixture was filtered and the filtrate concentrated in vacuo. The residue was dissolved in EtOAc and hexanes added until solid precipitated. After filtration, the title compound (1.2 g, 98%) was obtained as a light brown solid. [REF: BRISTOL-MYERS SQUIBB CO – WO2007/53452, 2007, A1]
EXAMPLE 3: To a solution of the amine (2.81 g, 10 mmol) in dioxane (45 mL) was added the chloride (2.57 g, 9.55 mmol), XantPhos (231 mg, 0.40 mmol), and t-BuONa (1.44 g, 15 mmol). Argon was bubbled through the mixture for 10 min. Pd2(dba)3 (183 mg, 0.20 mmol) was added, and argon was again bubbled through the mixture for 5 min. The reaction mixture was stirred at 100 C for 3 h, after which time it was cooled to 40 C and diluted with DCM (90 mL) and treated with Si-triamine (functionalized silica gel, 2.8 g) overnight at RT. Celite (6 g) was added and the mixture was filtered and the solid was rinsed with DCM (100 mL). The filtrate was concentrated to 25 mL and diluted with 4:1 EtOAc/hexane (20 mL). The resulting slurry was stirred at RT for 5 h, filtered, and the filter cake washed with 1:1 EtOAc/hexane (20 mL). The solids were air dried for several hours to provide the product as an off-white solid. [4.90 g, 100%] [REF: World Intellectual Property Organization WO 2012/129344 AI]

REFERENCES:
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako
- Angew. Chem. Int. Ed. 2019, 58, 17118 – 17129
- Org. Process Res. Dev. 2019, 23, 1478−148