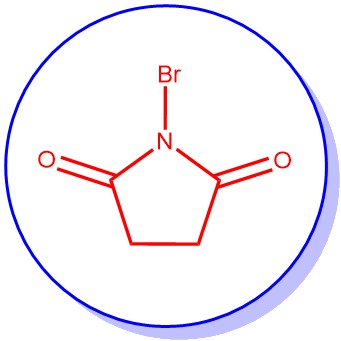
Introducing a bromine substituent at the allylic positions of olefins or at the benzylic positions of alkylated aromatic or heteroaromatic compounds is known as the Wohl-Ziegler Bromination reaction. The procedure utilizes N-Bromo succinimide (NBS) as a typical brominating agent in refluxing carbon tetrachloride (CCl4) in the presence of a radical initiator such as dibenzoyl peroxide [C6H5CO-OO-COC6H5], di-t-butylperoxide [(CH3)3C-OO-C(CH3)3], 2,2’-azobis(isobutyronitrile) [AIBN].
When the olefin has two allylic positions, the bromination is regioselective and favors the bromination of the more substituted position (the more stable allylic radical).
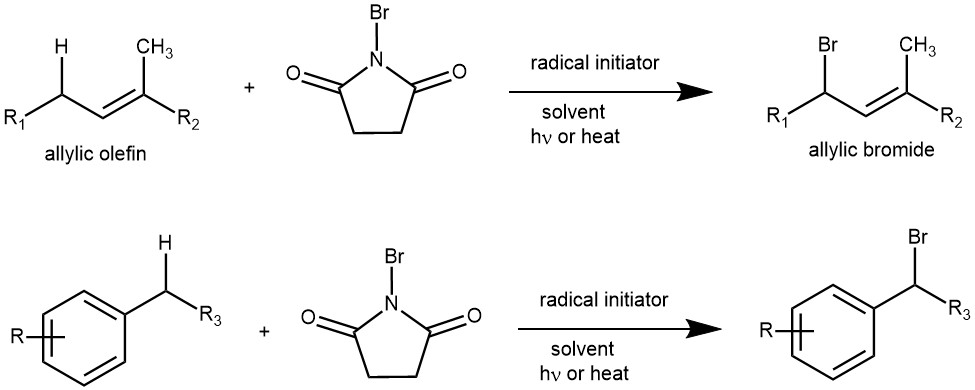
NOTE: If NBS is used in polar acid solvents such as acetic acid or aqueous sulfuric acid, the acid-catalyzed process predominates, and aromatic hydrocarbons are brominated at the aryl nucleus rather than at benzylic positions.
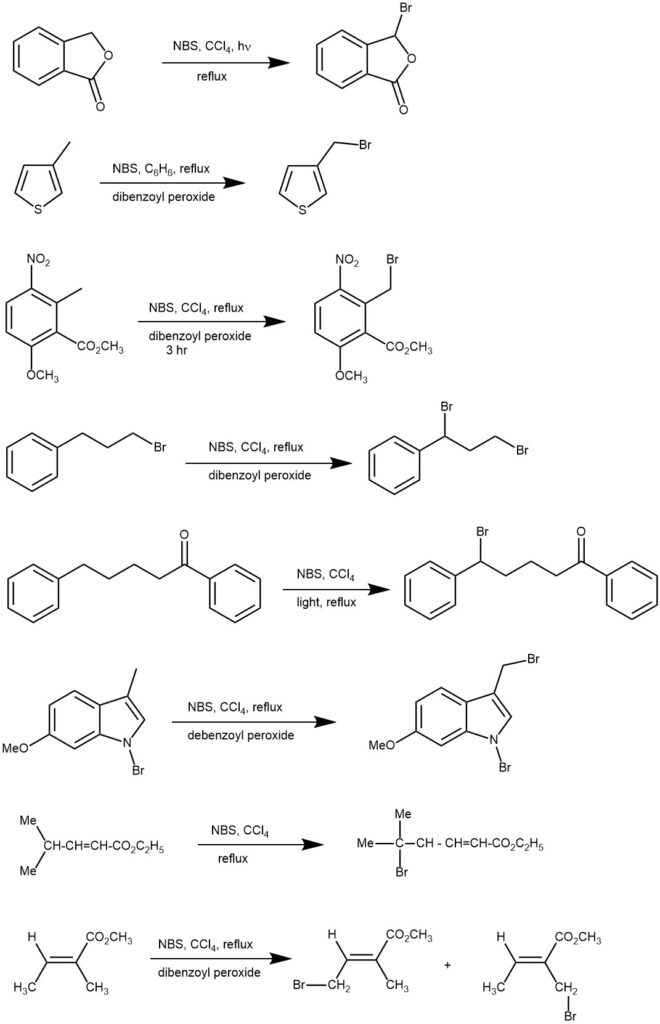
MECHANISM: Allylic and benzylic bromination with NBS proceeds by a free-radical chain mechanism. The radical initiator (e.g., AIBN) undergoes homolytic fission on heating or irradiation which reacts with bromine (Br2) to generate the bromine radical (Br∙). The bromine radical abstracts hydrogen radicals from the allylic or benzylic positions of the substrate to form the corresponding radical and HBr. This allylic or benzylic radical attacks the Br2 to form the allyl- or benzyl-brominated product and regenerate the bromine radical to repeat the cycle. The bromine molecule (Br2) necessary initially is almost always present in small quantities in NBS. Later on, Br2 is regenerated by the ionic reaction of NBS with the HBr as a by-product.

EXAMPLE 1: In a 500-mL round-bottomed flask fitted with a stirrer, nitrogen inlet tube, and reflux condenser are placed 40 g. (0.41 mole) of 2-heptene, 48.1 g (0.27 mole) of N-bromosuccinimide, 0.2 g. of benzoyl peroxide, and 250 mL of carbon tetrachloride. The reaction mixture is stirred and heated under reflux in a nitrogen atmosphere for 2 hours. The succinimide is removed by suction filtration, washed twice with 15-mL portions of carbon tetrachloride, and the carbon tetrachloride washings are combined with the filtrate. The carbon tetrachloride solution is transferred to a 500-mL Claisen flask modified so that the distilling arm carries a 25 × 300 mm section packed with glass helices. The capillary is attached to a source of nitrogen and the carbon tetrachloride is removed at 36–38°/190 mm.
The residue is transferred to a 125-ml. Claisen flask modified so that the distilling arm carries an 18 × 180 mm. section packed with glass helices. Nitrogen is led into the capillary, and, after a forerun of 1–3 g., there is collected 28–31 g. (58–64%) of 4-bromo-2-heptene, b.p. 70–71°/32 mm., n25D 1.4710–1.4715. A residue of 7–10 g. remains in the distilling flask.[REF: Org. Synth. 1958, 38, 8; DOI: 10.15227/orgsyn.038.0008]
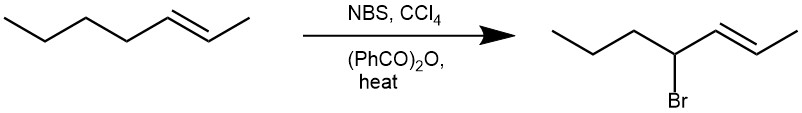
EXAMPLE 2: To a solution of methoxyimino-o-tolyl-acetic acid methyl ester (3.00 g, 14.49 mmol) in o-dichlorobenzene (50 mL), NB (5.16 g, 28.98 mmol) and AIBN (95 mg, 0.58 mmol) was added sequentially and the mixture was heated with a magnetic stirring at 80 ℃ for 8 h. Toluene (100 mL) was added to the cooled (room temperature) reaction mixture. The progress of the reaction was monitored by TLC; Rf of the starting compound (1) = 0.3, Rf of the product = 0.25. When the reaction mixture was heated for 8 h, the starting material disappeared on TLC and the reaction completed. The mixture was concentrated under reduced pressure and the residue was subjected to silica gel column chromatography (eluant, ethyl acetate : hexane (1 : 10, v/v) to give the desired product as a pale-yellow oil (3.81g, 92%) [REF: Clean Technol., Vol. 22, No. 4, December 2016, pp. 269-273; DOI: 10.7464/ksct.2016.22.4.269]

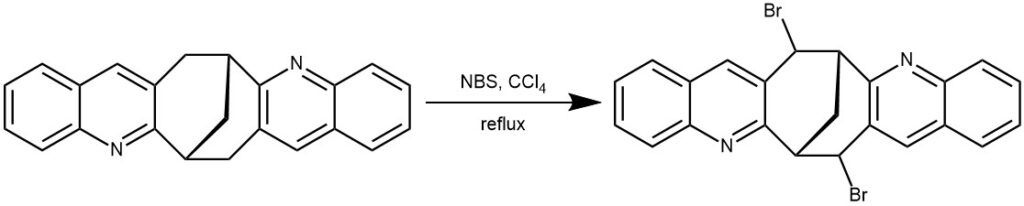
EXAMPLE 3: A solution of N-bromosuccinimide (0.33 g, 1.84 mmol) and the diquinoline (0.25 g, 0.74 mmol) in carbon tetrachloride (40 mL) was refluxed overnight. Succinimide was filtered from the cooled mixture, washed with a small amount of CCl4, and then the combined filtrate evaporated under reduced pressure to give a light yellow precipitate. Crystallization from ethyl acetate gave the colorless dibromide 10 (0.75 g, 82%), mp158-160 °C.[REF: dx.doi.org/10.1021/cg2006937 | Cryst. Growth Des. 2011, 11, 4474–4483]
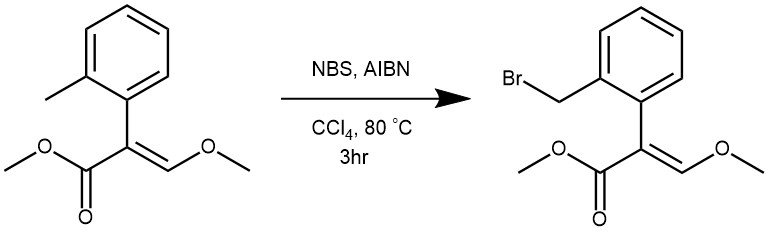
EXAMPLE 4: To a solution of (E)-methyl-2-(methylphenyl)-3-methoxyacrylate (1 g, 4.8 mmol) in carbon tetrachloride (10 mL), azaisobutyronitrile (AIBN, 0.078 g, 0.48 mmol) and N-bromosuccinamide (1.02 g, 5.76 mmol) was added. The reaction mixture was refluxed for 3 h. After completion of reaction (checked by TLC), carbon tetrachloride was removed under reduced pressure and the residure was extracted into ethyl acetate. Ethyl acetate was sequentially washed with 2*25 mL of water, 25 mL brine and dried over anhydrous Na2SO4. The solvent was then evaporated to get crude product, which on purification by column chromatography using ethyl acetate and petroleum ether as eluent afforded 1.31 g (95%) of the product. [REF: Indian Journal of Chemistry-Section B Organic and Medicinal Chemistry, 2015, 54B, 7, 908-917]
REFERENCES:
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako
- Modern Synthetic reactions by Herbert O. House
- Wohl–Ziegler bromination. (2022, April 29). In Wikipedia. httpWohl–Ziegler bromination. (2022, April 29). In Wikipedia. https://en.wikipedia.org/wiki/Wohl%E2%80%93Ziegler_brominations://en.wikipedia.org/wiki/Wohl%E2%80%93Ziegler_bromination