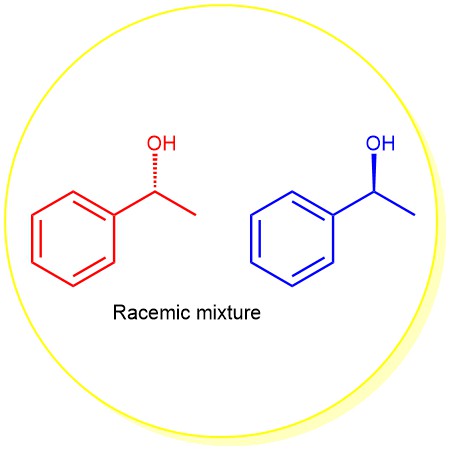
An equimolar mixture of two enantiomers is called a racemic modification (or racemate or racemic mixture). When enantiomers are mixed together in equal proportion, the rotation caused by a molecule of one isomer is exactly canceled by an equal and opposite rotation caused by a molecule of its enantiomer. As a result, the racemic modifications are optically inactive. The prefix ± or ‘dl’ is used to specify the racemic modification.
The enantiomers constituting the racemic modification pose a challenge to their separation using conventional methods. This is due to the fact that they share identical physical properties, rendering typical techniques ineffective. Fractional distillation is ineffective because their boiling points are indistinguishable. Fractional crystallization cannot be utilized because their solubilities in a particular solvent are also identical. Furthermore, chromatography cannot be employed since they are equally retained on a specific adsorbent. To recap, the enantiomers cannot be isolated from the racemic modification through regular means because they exhibit identical physical characteristics
The separation of a racemic modification into the constituent enantiomers is called the resolution of the racemic modification. The resolution processes can be classified in general into mechanical methods, chiral resolution (formation of diastereomers), enzymatic resolution, and kinetic resolution.
MECHANICAL METHOD OF RESOLUTION: If the enantiomers are solids, they are separated based on their differences in shape. This method is fairly simple but very tedious. Louis Pasteur carried out the first resolution of sodium ammonium tartrate using his hand lens and tweezers.
CHIRAL RESOLUTION (resolution by means of diastereomeric salt formation): In a chiral resolution, the interaction of the racemic mixture with a pure enantiomer forms diastereomers. These are then separated owing to the difference in their physical properties (the diastereomers have different physical properties). This chemical resolution method is successful because the diastereomers thus formed are different compounds, have different physical properties, and often can be separated by physical means (like fractional crystallization or column chromatography) and purified. The final step in this scheme is the chemical conversion of the separated diastereomers back to the individual enantiomers.
For example, when racemic organic acid (±)-HA reacts with an optically pure base, let’s say a levorotatory base, (-)-B, this will result in the formation of crystals of two different salts which are diastereomeric. These diastereomeric salts have different physical properties and can be separated by fractional crystallization.
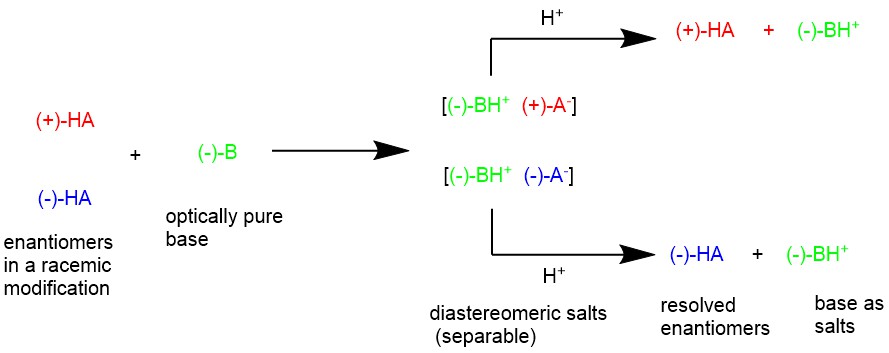
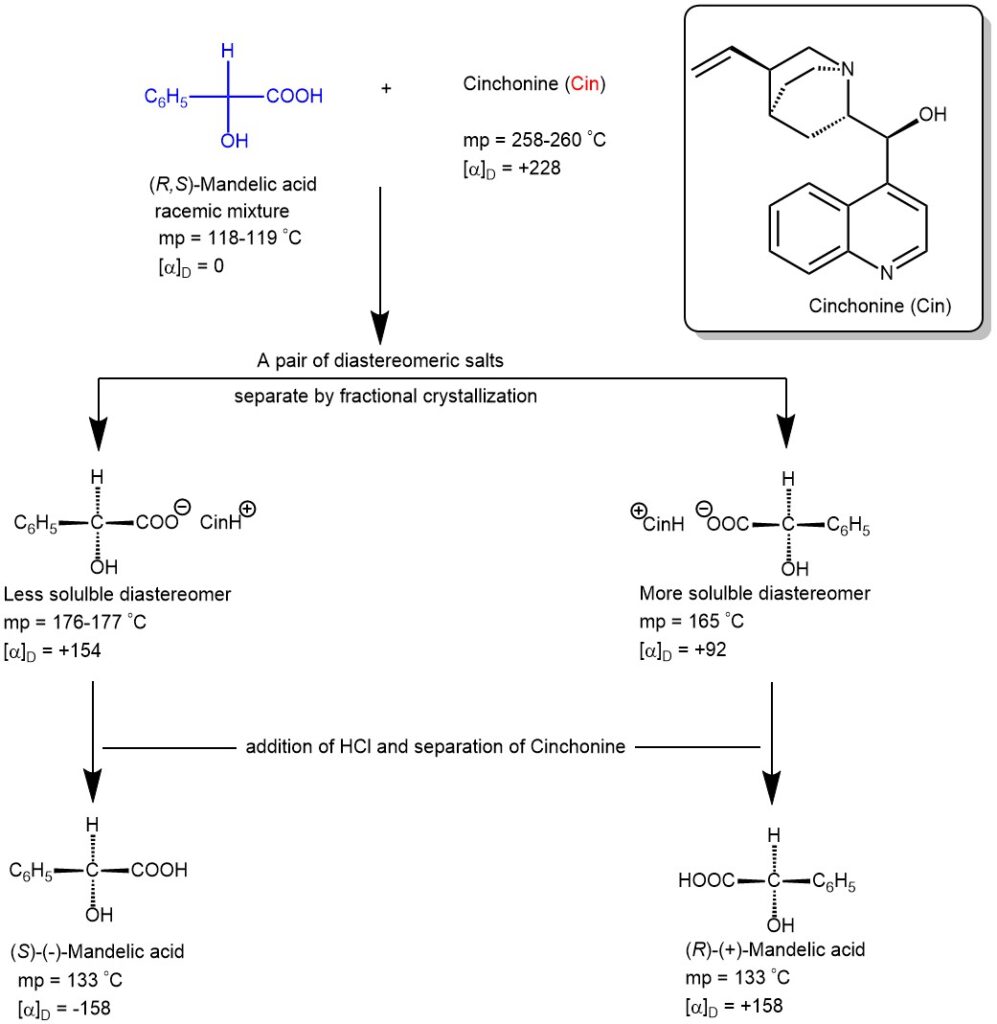
For a specific example, the resolution of a racemic mandelic acid by diastereomeric salt formation with the natural chiral base (+)-cinchonine is illustrated below. Racemic (±)-mandelic acid and optically pure (+)-cinchonine is dissolved in boiling water forming a pair of diastereomeric salts. When this solution is cooled, the less soluble diastereomeric salt crystallizes first which is filtered, collected, and further purified by recrystallization. This purified salt is then treated with aqueous HCl to precipitate the nearly pure enantiomer of mandelic acid.
ENZYMATIC RESOLUTION: Enzymes serve as catalysts and are specialized chiral protein molecules. They exhibit stereospecificity, meaning they exclusively interact with one enantiomer within a racemic mixture due to their own chirality. The enantiomer that forms a bond with an enzyme undergoes a chemical reaction, whereas the other enantiomer remains unaffected. Upon completing this process, one-half of the initial mixture is retained while the other half is discarded or reused.
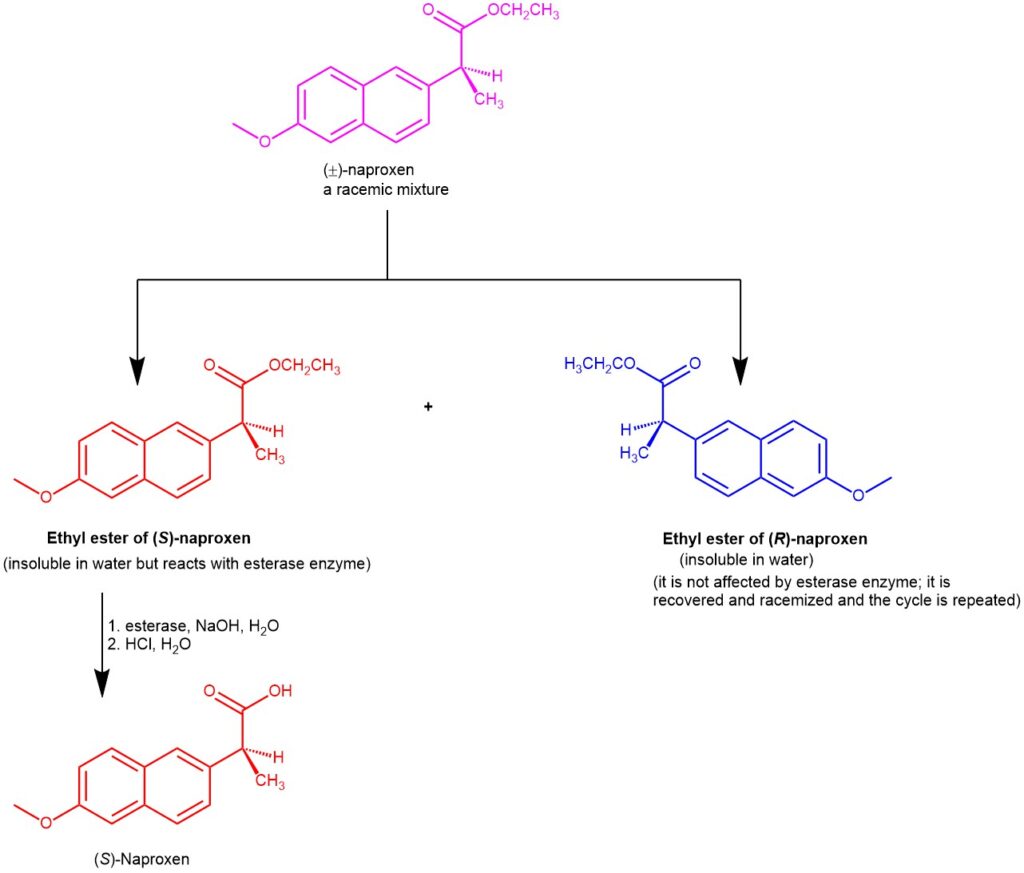
A class of enzymes called “esterase” catalyzes the hydrolysis of esters to give alcohol and carboxylic acid. The ethyl ester of (S)-naproxen and (R)-naproxen crystallize in two enantiomeric crystal forms and are insoluble in water. Esterase in an alkaline solution selectively hydrolyzes the (S)-naproxen ethyl ester to the (S)-naproxen, which goes into the aqueous solution as the sodium salt while the (R)-naproxen ethyl ester remains unaffected. Filtering the aqueous alkaline solution recovers the crystals of (R)-naproxen ethyl ester. The alkaline solution containing the (S)-isomer is acidified to give enantiomerically pure (S)-naproxen.
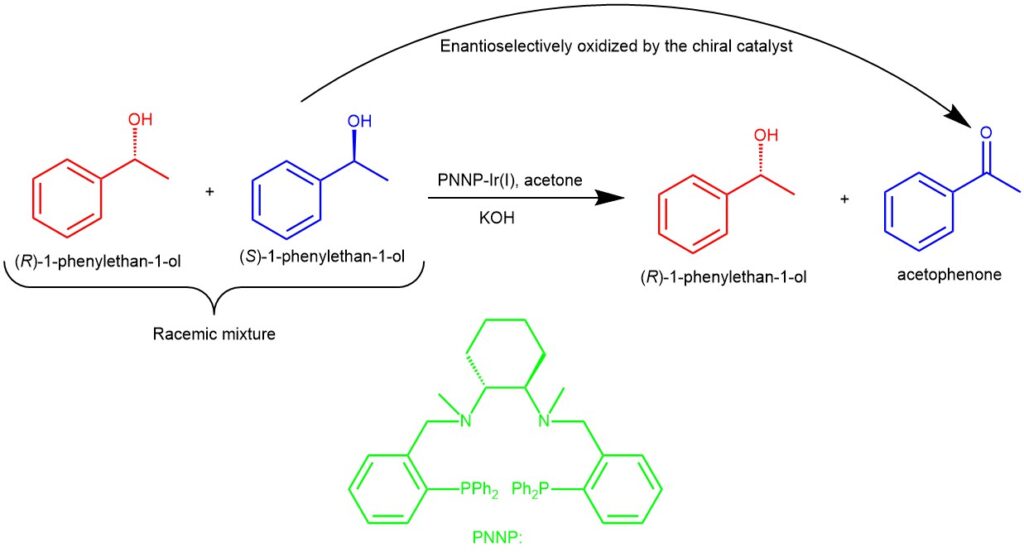
KINETIC RESOLUTION: In the kinetic resolution, the separation of enantiomers is based on the difference in reaction rates of enantiomers with a chiral catalyst. Unlike the former, kinetic resolution takes advantage of the difference in the chemical properties of the starting materials in the racemic mixture.
For example, Chiral diaminodiphosphine-Ir(I) complex was found to efficiently catalyze enantioselective oxidation of racemic secondary alcohol (1-phenyl ethanol) in acetone. In the presence of a base (KOH), oxidative kinetic resolution of the alcohol proceeded smoothly with excellent enantioselectivity. The chiral PNNP-Ir(I) acts as a versatile catalyst for the enantioselective oxidation of (S)-1-phenyl ethanol to acetophenone leaving behind the (R)-1-phenyl ethanol
CHIRAL CHROMATOGRAPHY: A common method of resolving enantiomers today is chromatography using a chiral column packing material. Each enantiomer interacts differently with the chiral molecules of the packing material, and the elution time will be different for the two enantiomers. A wide variety of chiral column packings have been developed for this purpose.
References:
- Organic chemistry, 5th edition by William H. Brown, Christopher S. Foote, Brent L. Iverson, and Eric V. Anslyn.
- Org. Lett., Vol. 8, No. 24, 2006
- Organic chemistry, 7th edition by Robert T. Morrison, Robert N. Boyd, and Saibal K. Bhattacharjee
- https://www.masterorganicchemistry.com/2017/02/24/optical-purity-and-enantiomeric-excess/