LATEST POSTS
organic reactions and mechanisms
October 22, 2023
Chitranjan
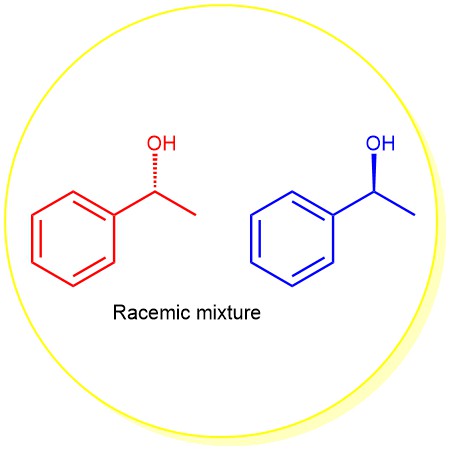
An equimolar mixture of two enantiomers is called a racemic modification (or racemate or racemic mixture). When enantiomers are mixed together in equal proportion, the rotation caused by a molecule of...
October 1, 2023
Chitranjan
FISCHER PROJECTION FORMULA: It is a two-dimensional depiction of an organic molecule at the tetrahedral carbon center. The four bonds at the tetrahedral carbon are represented by two vertical and two horizontal...
September 24, 2023
Chitranjan
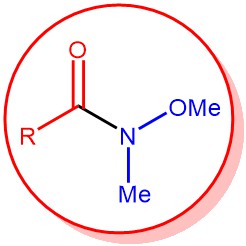
The synthesis of ketones from N-methoxy-N-methyl amide, called as Weinreb amide, using organometallic reagents is known as the Weinreb ketone synthesis. Generally, the direct conversion of acid chlorides...
September 10, 2023
Chitranjan
The direct conversion of carboxylic acids or acid chlorides or esters to ketones or aldehydes using organometallic reagents usually does not result in high yield because the intermediate ketones are highly...
September 10, 2023
Chitranjan
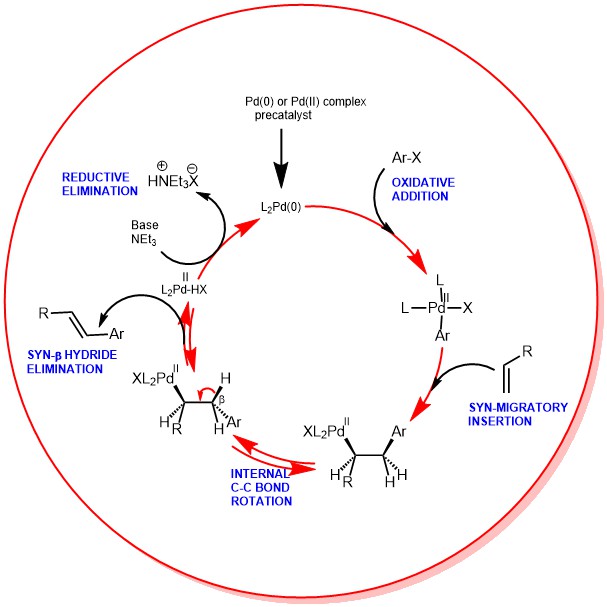
During the early 1970s Tsutomu Mizoroki and Richard F. Heck independently discovered that the reaction of aryl, benzyl, and styryl halides with alkenes at a high temperature in the presence of a hindered...
September 5, 2023
Chitranjan
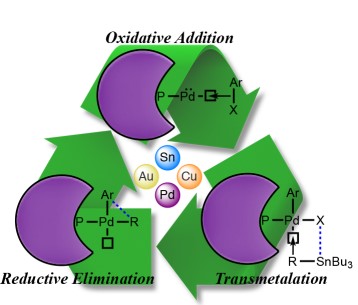
In 1976, the first palladium-catalyzed cross-coupling of organotin compounds was accomplished by C. Eaborn, et al. The next year, M. Kosugi and T. Migita described the transition metal-catalyzed cross-coupling...
September 4, 2023
Chitranjan
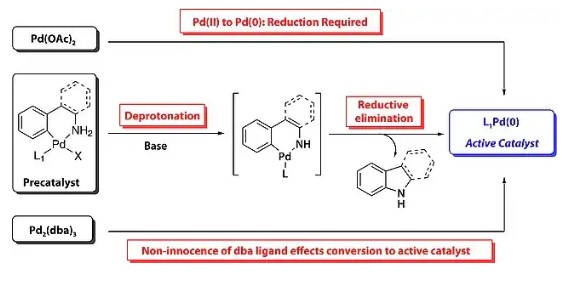
Aryl amines and related heterocycles are essential components in natural products, pharmaceuticals, agrochemicals, and various other compounds. This significance has driven chemists to seek advanced methods...
August 28, 2023
Chitranjan
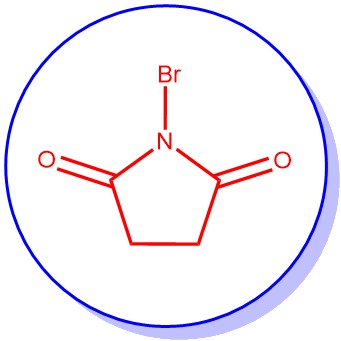
Introducing a bromine substituent at the allylic positions of olefins or at the benzylic positions of alkylated aromatic or heteroaromatic compounds is known as the Wohl-Ziegler Bromination reaction. The...
August 28, 2023
Chitranjan
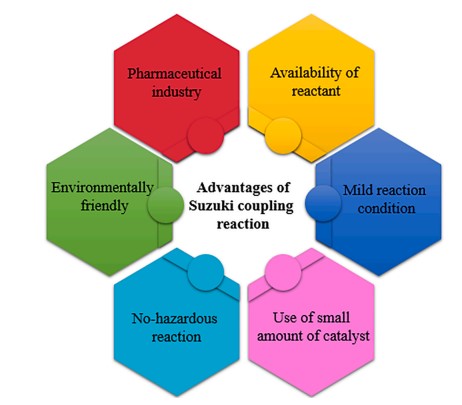
Cross-coupling reactions occur when two reagents react together with a metal catalyst to form a new covalent bond. A cross-coupling reaction in organic synthesis occurs when two fragments are joined together...
August 14, 2023
Chitranjan
An indirect method for the selective alkylation and acylation of an aldehyde or ketone is via an intermediate called ‘Enamine’. An enamine is an unsaturated compound derived from the condensation of an...
No posts found