Cross-coupling reactions occur when two reagents react together with a metal catalyst to form a new covalent bond. A cross-coupling reaction in organic synthesis occurs when two fragments are joined together with the aid of a metal catalyst. Cross-coupling has been an essential reaction in catalytic organic chemistry with the pioneering work by Heck, Negishi, and Suzuki who were awarded the Nobel Prize in Chemistry in 2010 for Palladium-catalyzed cross-coupling
Among the various types of Cross-coupling reactions, the Suzuki-Miyaura-usually called Suzuki coupling is one of the most widely utilized reactions. It was discovered in 1979 by A. Suzuki and N. Miyaura.
The Suzuki reaction is the coupling of an aryl or vinyl boronic acid with an aryl or vinyl halide or triflate using a palladium catalyst. It is a powerful cross-coupling method that allows for the synthesis of conjugated olefins, styrenes, and biphenyls. Suzuki coupling reaction is performed between an organic halide or triflate as an electrophile and organoborane as a nucleophile with a transition metal catalyst, suitable base, aqueous and organic solvents, and temperature to make biaryl products.

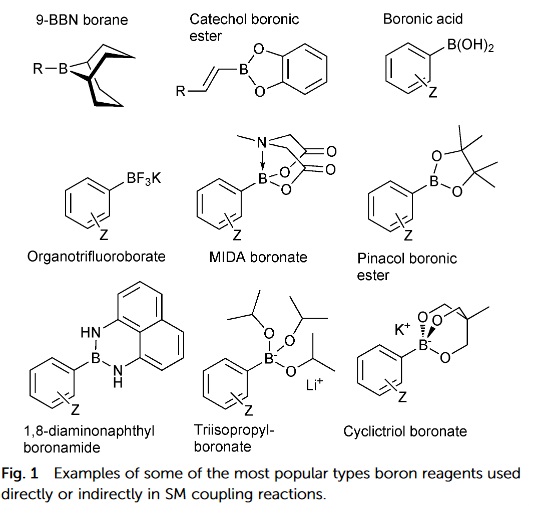
The organoboron compounds used for this reaction include boronic acids, esters, and trifluoroborate salts. Even the less reactive alkyl boronic acids can be considered for this reaction.
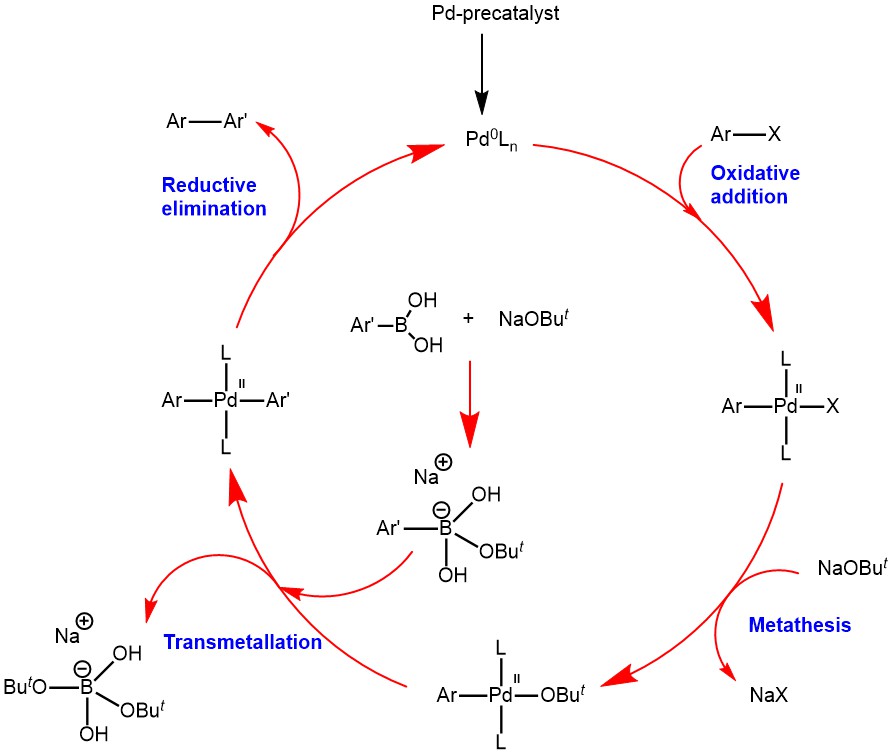
MECHANISM: The mechanism of Suzuki-coupling consists of the following steps:
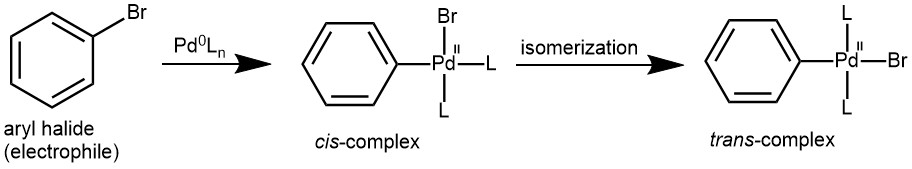
OXIDATIVE OXIDATION: In this step, aryl halide (the electrophile) couples with a Palladium (Pd) catalyst to form an organopalladium. In this step, the oxidation number of Pd changes from (0) to (II). Oxidative addition initially gives a cis-complex that rapidly isomerizes to its trans-isomer.
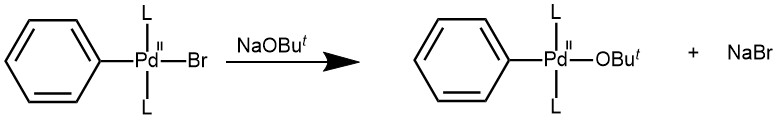
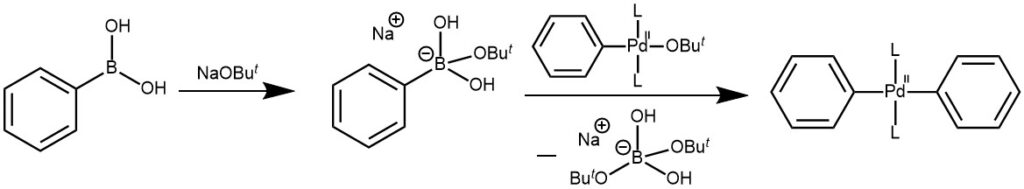
METATHESIS: The second step involves the exchange of anion attached to the palladium for the anion of the base.
TRANSMETALLATION: This step involves the transfer of ligand from the organoboron compound to the palladium complex formed in the metathesis. Organoboron compounds (weak nucleophiles) are highly covalent in character and do not undergo transmetallation readily in the absence of a base. The base thus converts the weakly nucleophilic organoboron to a strong nucleophile. The quaternization of the boron atom with the base increases the nucleophilicity of the alkyl group and accelerates its transfer to the palladium in the transmetallation step.
REDUCTIVE ELIMINATION: This step regenerates the Pd0 catalyst and forms the C-C sigma bond in the product. Isomerization to the cis complex is required before reductive elimination can occur.
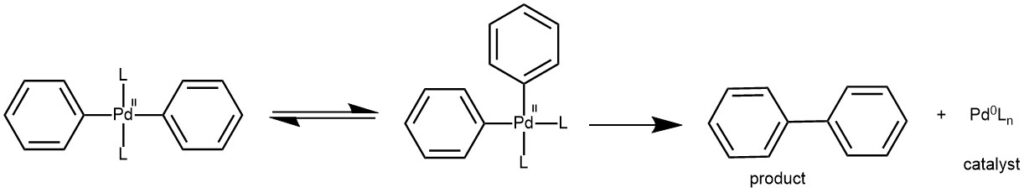
A general mechanism for aryl-aryl Suzuki-Miyaura coupling
EXAMPLE 1: To a solution of the Iodo compound (1.0 g, 1.66 mmol) and the boronic acid (392 mg, 2.49 mmol) in DMF (10 mL) was added K3PO4 (704 mg, 3.32 mmol). The mixture was purged with N2 for 15 min, after which time Pd(PPh3)4 (176 mg, 0.152 mmol) was added and the mixture was stirred at 85 C for 5 h. The mixture was then poured in ice cold H2O (50 mL) and extracted with EtOAc. The combined organics were washed with H2O, dried, concentrated, and purified by silica gel chromatography (eluting with 15% EtOAc/hexane) to provide the product as a brown solid. [0.52 g, 53%][REF: World Intellectual Property Organization WO2012129338]

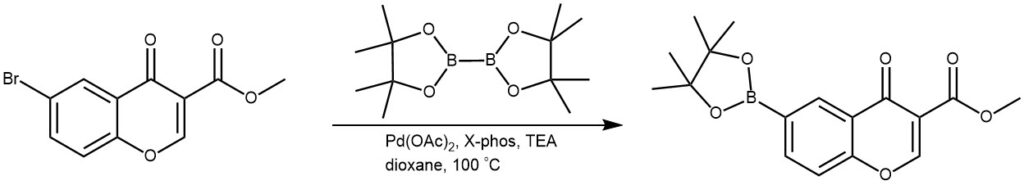
EXAMPLE 2: A mixture of the SM (400 mg, 1.41 mmol), bis(pinacolato)diboron (1 g, 3.94 mmol), XPhos (135 mg, 0.28 mmol), Pd(OAc)2 (16 mg, 0.06 mmol), and TEA (570 mg, 5.6 mmol) in dioxane (20 mL) was degassed and refluxed at 100 C for 20 min under N2. The solvent was removed in vacuo, the residue diluted with DCM (20 mL), washed with H2O (2 x 20 mL), then brine. The org layer was dried (Na2SO4), concentrated, and recrystallized with DCM/hexane to provide the product[300 mg]. [REF: World Intellectual Property Organization WO2010038081]
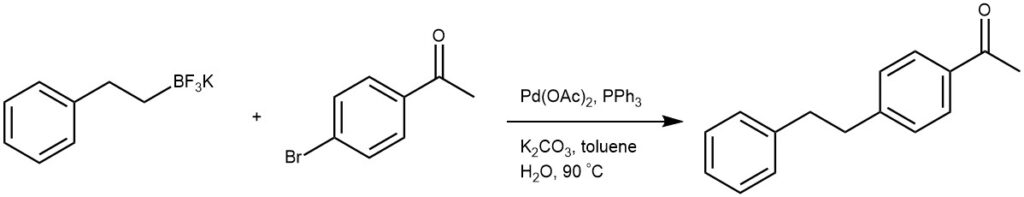
EXAMPLE 3: A three-necked, 250-mL round-bottomed flask, equipped with a reflux condenser, a rubber septum, a glass stopper, a magnetic stirring bar, and connected to a vacuum-argon manifold, is charged sequentially with potassium 2-phenethyltrifluoroborate (7.77 g, 36.6 mmol, 1.05 equiv), 4-bromoacetophenone (6.97 g, 35.0 mmol), potassium carbonate (14.5 g, 105 mmol, 3.0 equiv), triphenylphosphine (0.275 g, 1.05 mmol, 0.03 equiv) and palladium acetate (0.118 g, 0.53 mmol, 0.015 equiv) (Note 10). The flask is evacuated and filled with dry argon three times. Toluene (105 mL) and H2O (21 mL) are added, and the resulting two-phase mixture is stirred in a 95 °C oil bath until consumption of the 4-bromoacetophenone is indicated by GC analysis. The mixture is cooled to room temperature and then is transferred to a 250-mL separatory funnel. The flask is rinsed with toluene (20 mL) and H2O (20 mL). The layers are shaken and separated. The organic layer is washed with 10% aq. citric acid solution (20 mL) (Note 13) and saturated aq. NaCl solution (20 mL). The organic layer is dried over anhydrous Na2SO4 (0.780 g) and filtered through a medium porosity glass-fritted funnel, rinsing the drying agent with toluene (20 mL).
The resulting solution is concentrated (50 °C water bath, 9 mmHg) to a light yellow solid, which is crystallized from methanol to give 5.79 g (74%, two crops) of a light tan solid. Recrystallization of 0.3 g of this solid from hot methanol (2 mL) provided white, crystalline material (0.25 g) of analytical purity.[REF: Organic Syntheses, Vol. 84, p. 317-324 (2007); Coll. Vol. 11, p. 289-295 (2009)]
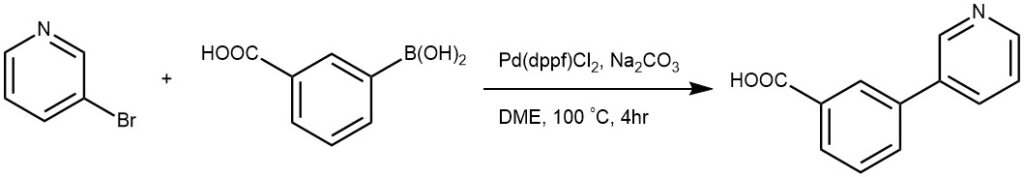
EXAMPLE 4: To a solution of 2-bromopyridine(166 mg, 1 mmol) in DME (12 mL) and 2 N Na2CO3 (4 mL)was added 3-Carboxyphenylboronic acid (237 mg, 1.5 mmol), [1,1′-bis(diphenylphosphino)ferrocene]-dichloropalladium(II) (73 mg,0.1 mmol). The reaction mixture was protected by N2 and heated to100 °C for 4 h. The mixture was then cooled to rt, filtered and concentratedin vacuo. The residue was purified by silica gel chromatography(DCM/MeOH = 20:1) to produce the title compound (4.148 mg, 74%) as a colorless oil. [REF: [Bioorganic Chemistry, 2020, vol. 101]
REFERENCES:
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako
- Suzuki reaction. (2023, July 13). In Wikipedia. https://en.wikipedia.org/wiki/Suzuki_reaction
- Chem. Soc. Rev., 2014, 43, 412
- Molecules 2020, 25, 3493; doi:10.3390/molecules25153493
- Polyhedron 227, (2022), 116124