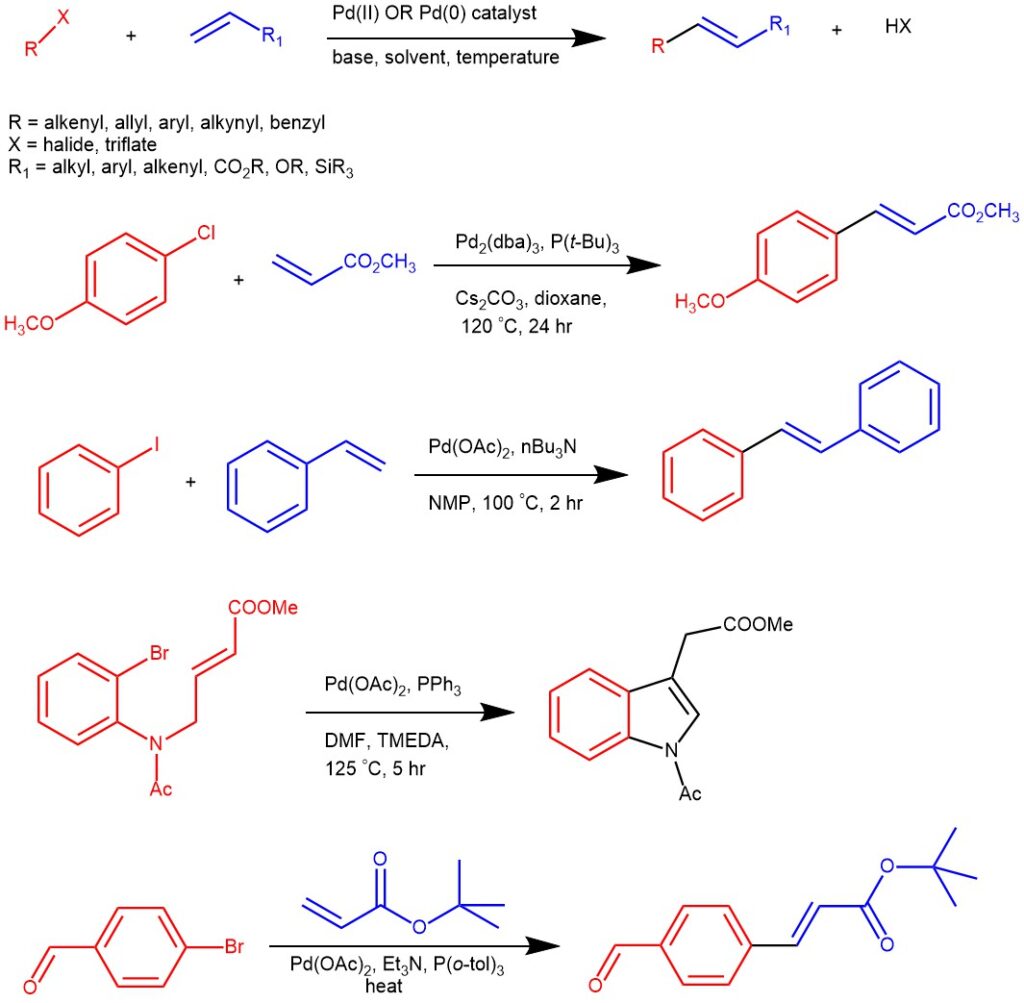
During the early 1970s Tsutomu Mizoroki and Richard F. Heck independently discovered that the reaction of aryl, benzyl, and styryl halides with alkenes at a high temperature in the presence of a hindered amine base and palladium catalyst resulted in the equivalent substituted alkenes. The Palladium-catalyzed arylation or alkenylation of alkenes is known as the Heck reaction. The Heck reaction is one of the most studied coupling reactions and was recognized with the Nobel Prize in Chemistry in 2010.
The aryl halide variants in addition to typical aryl bromides, and iodides are aromatic triflates, aroyl chlorides, aryl sulfonyl chlorides, aromatic diazonium salts, aroyl anhydrides, aryl chlorides and aryl silanols. The reaction conditions tolerate a wide range of functional groups on the olefin component like esters, carboxylic acids, nitriles, phenols, dienes, etc.
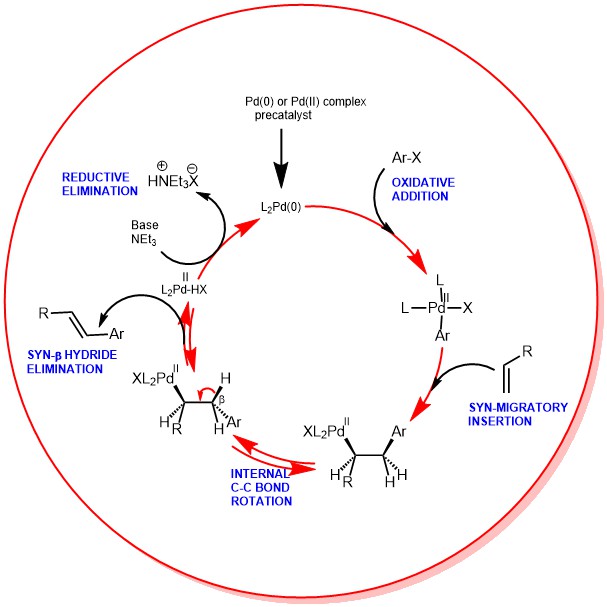
MECHANISM: The active Palladium catalyst is generated in situ from suitable precatalysts like Pd (OAc)2 and Pd(PPh3)4 and the reaction is usually conducted in the presence of monodentate or bidentate ligands and a base. The bases used are both soluble and insoluble like:

The oxidative addition involves the rupture of the Ar-X bond and the formation of the Pd-Ar and Pd-X bonds. This also results in the oxidation of the Palladium catalyst from Pd(0) to Pd(II) states.
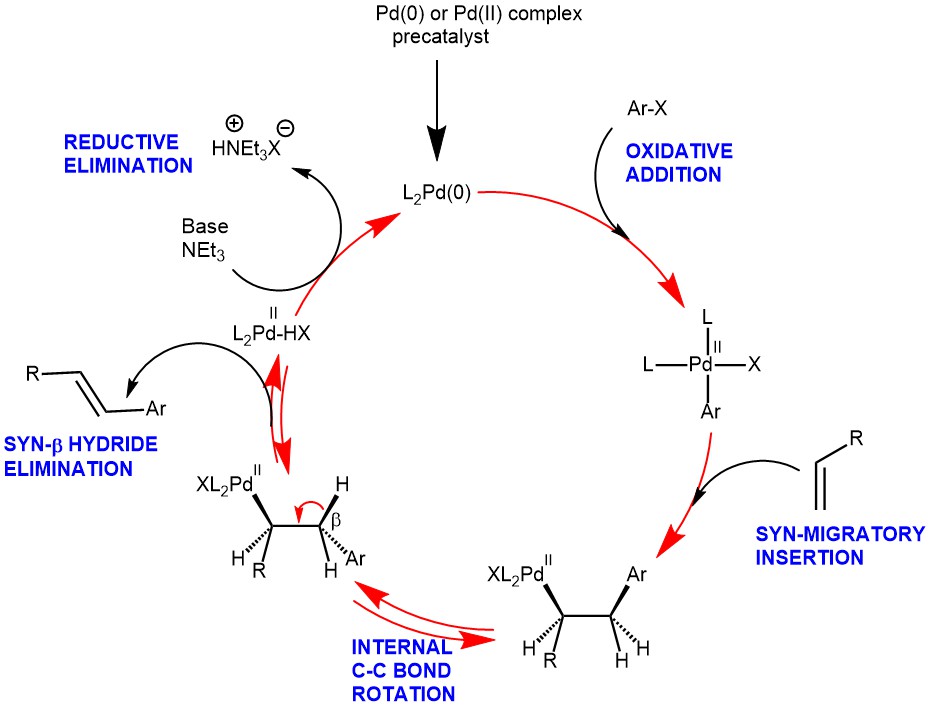
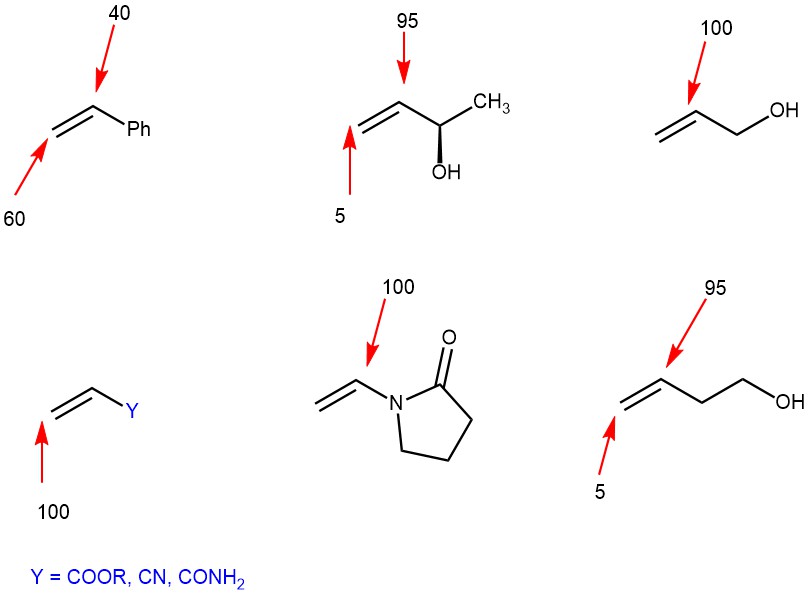
Migratory insertion is the actual C-C bond-forming step in the Heck-catalytic cycle. This step proceeds with syn-stereochemistry. It is this step which is responsible for regio– and stereoselectivity. For neutral Palladium complexes, the regiochemistry is governed by steric ie., substitution occurs at the least substituted olefinic carbon. Thus, the position of Aryl attachment follows:

However, when cationic Pd-complexes are used, regiochemistry is affected by electronics. The cationic Pd-complexes increase the polarization of the alkene favoring the transfer of the vinyl or aryl group to the site of less electron density.
The β-hydride elimination step is where Palladium hydride is eliminated to release the double bonded product. This step also proceeds with syn–stereochemistry. Reversible le β-hydride elimination can lead to alkene isomerization.
The β-hydride elimination is followed by the reductive elimination step. The addition of a base is required to reduce the L2PdHX complex back to the L2Pd(0) to continue the cycle.
EXAMPLE 1: To a solution of 3-bromobenzaldehyde, 168, (3.00 g, 16.2 mmol) in DMF (69 mL) under a dry nitrogen atmosphere, palladium acetate (73 mg, 0.32 mmol), tri-o-tolylphosphine (197 mg, 0.65 mmol), ethyl acrylate (2.20 mL, 20.3 mmol) and triethylamine (4.50 mL, 32.4 mmol) were added. The system was deoxygenated (toggle between vacuum and nitrogen five times), the mixture was heated to 125° C. for 19 hours and then cooled to room temperature. The reaction was poured into water and extracted with ether. The organic layer was washed with HCl (4N) and brine, dried over MgSO4 and filtered. The filtrate was concentrated under reduced pressure to give 169 (2.74g, 83%), which was used without further purification.[REF: PFIZER INC – US2004/63955, 2004, A1]

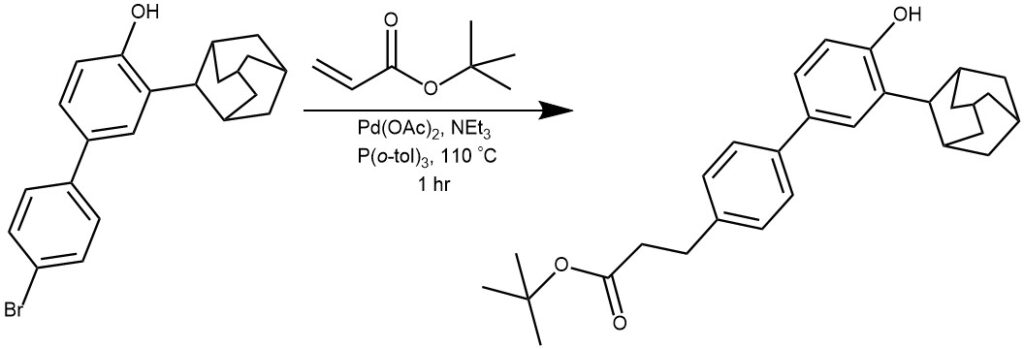
EXAMPLE 2: A round flask containing a mixture of 3-adamantan-1-yl-4′-bromobiphenyl-4-ol (4) (2.0 g, 5.22 mmol), tert-butyl acrylate (1.21 mL, 8.35 mmol), Pd(OAc)2 (11.7 mg, 0.0522 mmol) and tri-o-tolyl phosphine in 2.42 mL of Et3N was immersed in an oil bath and kept at 110 °C for 1 h. Iced water was then added, followed by 1 N HCl. The aqueous phase was extracted repeatedly with ethyl acetate, dried over Na2SO4, filtered, and evaporated. The crude product was purified by flash chromatography (hexane/ethyl acetate 85:15) to give 2.025 g of the title compound as a white solid. Yield 90%.[REF: Bioorganic and Medicinal Chemistry, 2012, vol. 20, # 7, p. 2405 – 2415]
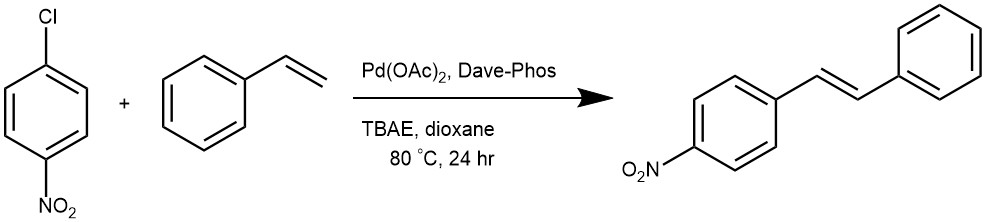
EXAMPLE 3: After standard cycles of evacuation and backfilling with dry and pure argon, an oven-dried Schlenk tube equipped with a magnetic stirring bar was charged with Pd(OAc)2 (2 mol %, 2.2 mg), Dave-Phos (6 mol %, 11.8 mg), the aryl chlorides if a solid (0.5 mmol, 1 equiv), and TBAE (1 mmol, 0.301 g). The tube was evacuated and backfilled with argon (this procedure was repeated three times). Under a counter flow of argon, styrene (0.75 mmol, 86 μL), and 1,4-dioxane (1.0 mL) were added by syringe. The tube was sealed, and the mixture was allowed to stir under argon at 80 °C for 24 h. Upon completion of the reaction, the mixture was diluted with ethyl acetate and filtered through silica gel (which was rinsed with EtOAc), and solvent was removed with the aid of a rotary evaporator. The residue was purified by column chromatography on silica gel and the product was dried under high vacuum for at least 0.5 h. [REF: dx.doi.org/10.1021/jo201196a |J. Org. Chem. 2011, 76, 8036–8041]
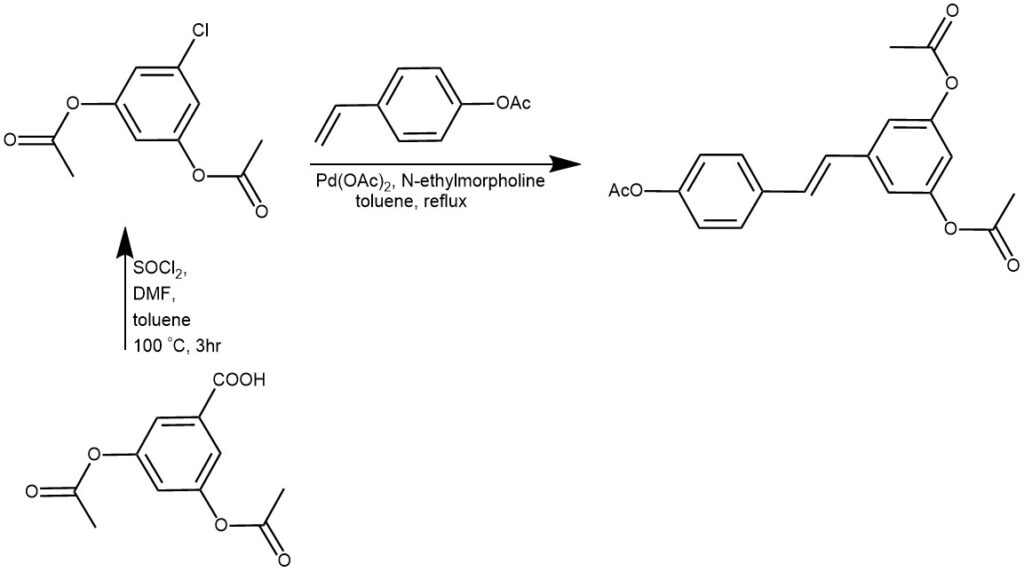
EXAMPLE 4: A suspension of 3,5-diacetoxybenzoic acid (8.022 g, 33.71 mmol) in a mixture of toluene (130 mL),DMF (500 μL) and thionyl chloride (16.00 mL, 220.6 mmol) was heated at 100 °C for three hours underan argon gas atmosphere. The solvents were removed by vacuum distillation and the residue re-suspendedin toluene (85 mL) and sonicated under vacuum to remove dissolved gases. 4-Acetoxystyrene (5.74 mL,37.5 mmol), N-ethylmorpholine (4.31 mL, 33.9 mmol) and palladium diacetate (35 mg, 0.16 mmol,0.46 mol %) were added and the reaction heated to reflux for 2 h. Further palladium diacetate (116 mg,0.52 mmol, 1.54 mol %) was added and the reaction left to reflux overnight. On return to roomtemperature, ethyl acetate (500 mL) was added, the solution washed with 0.1 M aq. HCl (2 × 300 mL)and water (300 mL) and then dried and evaporated to return a brown solid. Purification with columnchromatography (isocratically eluted with 2:1 Et2O/hexane) gave 7.888 g of a white solid, shown by1H-NMR spectroscopy to be predominantly the desired adduct. Further chromatography (gradienteluted starting with 4:1 hexane/EtOAc and finishing with 2:1 hexane/EtOAc) returned pure (E)-3,4′,5-triacetoxystilbene (6.071 g, 51%) as a white solid. Rf 0.29 (2:1 hexane/EtOAc)[REF: Molecules, 2015, vol. 20, # 9, p. 17601 – 17613]
REFERENCES:
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako
- Cabri, W.; Candiani, I. Acc. Chem. Res. 1995, 28, 2–7.
- Cabri, W.; Candiani, I.; Bedeschi, A.; Penco, S.; Santi, R. J. Org. Chem. 1992, 57, 1481–1486.
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Catalysis/Catalyst_Examples/Heck_Reaction
- Heck reaction. (2023, September 8). In Wikipedia. https://en.wikipedia.org/wiki/Heck_reaction