In 1976, the first palladium-catalyzed cross-coupling of organotin compounds was accomplished by C. Eaborn, et al. The next year, M. Kosugi and T. Migita described the transition metal-catalyzed cross-coupling of organotin compounds with aryl halides and acid chlorides. Following this, in 1978, J.K. Stille used organotin compounds to synthesize ketones using milder reaction conditions than those used by Kosugi but giving improved yields. In the early 1980s, Stille continued to develop and improve on his methodology, and today the palladium-catalyzed coupling reaction between an organostannane and an organic electrophile, like aryl halide, to form carbon-carbon bonds is known as the Stille cross-coupling reaction.
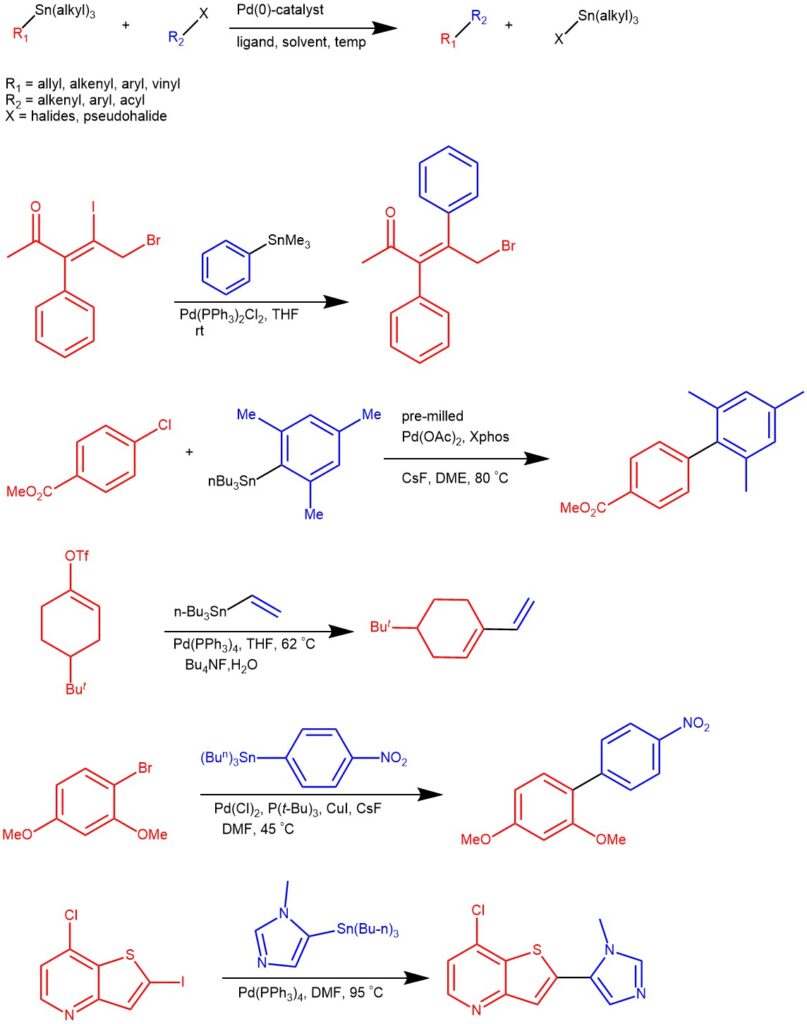
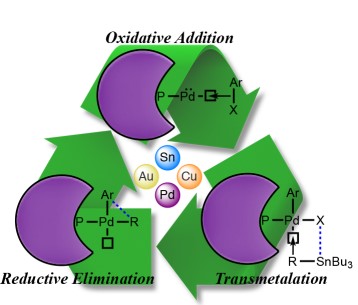
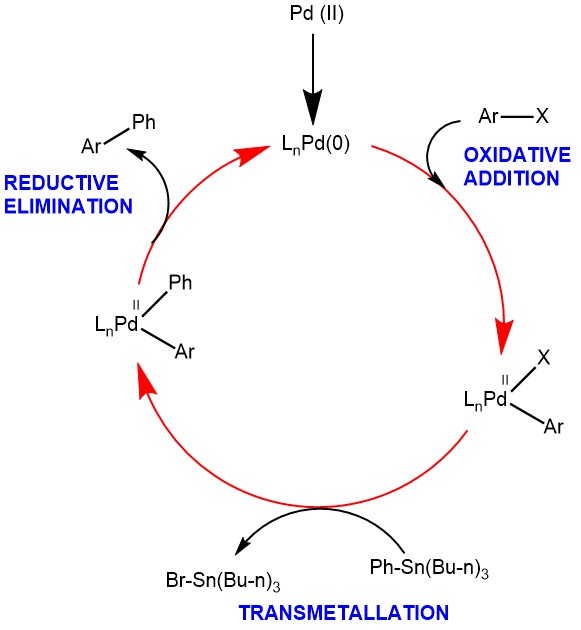
MECHANISM: In the simplest representation, the Pd-catalyzed Stille coupling reaction has three steps: oxidative addition, transmetallation, and reductive elimination. The oxidative addition of aryl halide to the Palladium (0) produces the 16-electron Pd(II) complex. The initial cis-complex rapidly isomerizes to the trans-complex. The transmetallation step involves the transfer of the alkyl or aryl group from the tin-coupling partner to the Pd(II) intermediate. Different groups migrate at different rates and the order of migration is alkynyl > vinyl > aryl > allyl ~ benzyl >> alkyl. The very slow migration rate of the alkyl substitution allows the transfer of aryl or vinyl groups when mixed organostannanes containing three methyl or butyl groups are used as coupling partners. The reductive elimination gives the coupled product and regenerates the Pd(0) catalyst.
It has been observed that the electron-rich and sterically hindered aryl halides undergo slow oxidative addition and electron-poor stannanes undergo slower transmetallation. Copper iodide and fluoride and sterically hindered, electron-rich ligands on Palladium typically accelerate the coupling process. Fluoride can coordinate with the organo-tin to form a hypervalent species that is believed to undergo transmetallation at a faster rate.
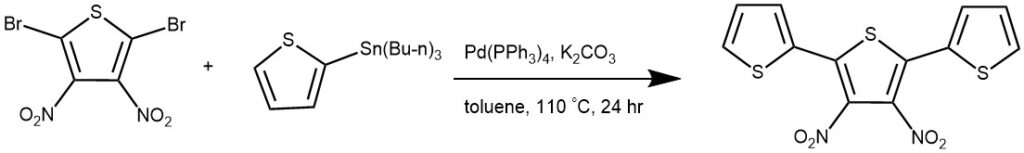
EXAMPLE 1: Tributyl(thiophen-2-yl)stannane (3.73 g, 10 mmol), 2,5-dibromo-3,4-dinitrothiophene (1.1 g, 3.33 mmol) and 77 mg Pd(PPh3)4 (1% mol) were put into the two-neck bottle, and the 100mL toluene was added. The mixture was refluxed for 24 h under N2 atmosphere. And then the reaction was stopped and cooled to room temperature. The mixture was first purified by column chromatography using THF as the eluent to get orange liquid. And then it was concentrated and purified by column chromatography using petroleum ether as the eluent to get orange solid. Yield: 80%. [REF: Polymer, 2013, vol. 54, # 22, p. 6191 – 6199]
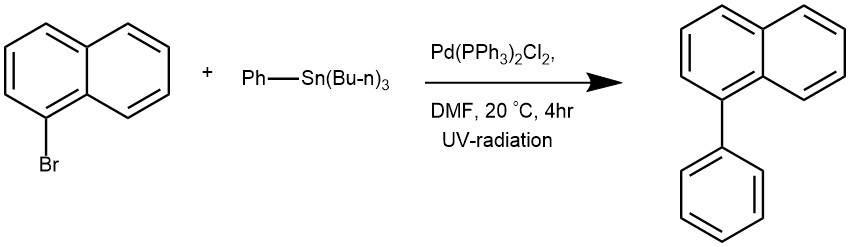
EXAMPLE 2: Aryl halide (1, 0.10 mmol) and a solution of PdCl2(PPh3)2 (0.01 mmol, 10 mol%) in DMF (2.34mL) were added to 13.5 mL glass vial containing a magnetic stirring bar. Aryl stannane (2, 0.30 mmol)and DMF (2.66 mL) were added to the mixture, and then the vial was sealed with PE (polyethylene)cap under air. The mixture was stirred under UV-light irradiation with 2×1.2 W UV-light (2 cm away,with cooling fan to keep the reaction room temperature) under air for 4 h. QuadraSilTM (100 mg) wasadded, and the resulting mixture was stirred for 2 min for quenching the reaction. After filtration(using a membrane filter) and evaporated, the residue was purified by preparative GPC withchloroform as eluent to afford the coupling product 3.[REF: Chemistry Letters, 2022, vol. 51, # 2, p. 124 – 126]
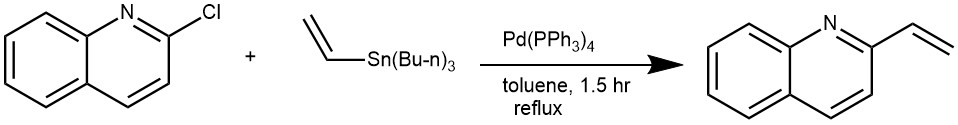
EXAMPLE 3: A solution of 2-chloroquinoline (1 g, 6.11 mmol) and vinyl tributyl tin (2.69 mL, 9.17 mmol) in toluene (30 mL) was treated with Pd(PPh3)4 (0.706 g, 0.611 mmol) and heated to reflux for 1.5 h. The reaction mixture was concentrated and the resulting material was purified directly by gradient elution on silica gel (0 to 25% EtOAc in hexanes) to afford the title compound as a colorless oil (941 mg, 99%).[REF: MERCK & CO INC – EP2714041, 2016, B1]
EXAMPLE 4: To a mixture of the bromide (500 mg, 2.00 mmol), CsF (668 mg, 4.40 mmol), P(n-Bu)3 in hexane (0.347 mL, 0.120 mmol), Pd2(dba)3 (36.6 mg, 0.040 mmol), and toluene (10 mL) was added the stannane (0.587 mL, 2.00 mmol). The reaction mixture was stirred at 80 C overnight, after which time was added a saturated KF solution. The resulting mixture was stirred 1 h, filtered, and the filtrate diluted with EtOAc. The org mixture was washed with H2O, dried (MgSO4), and concentrated. The resulting solids were purified by silica gel column chromatography (EtOAc/hexane) to provide the product. [450 mg] [REF: World Intellectual Property Organization WO2011017578]

REFERENCES:
- ACS Catal. 2015, 5, 5, 3040–3053
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako
- Stille Cross-Coupling Reaction | Thermo Fisher Scientific – US
- Stille Coupling – Chemistry LibreTexts