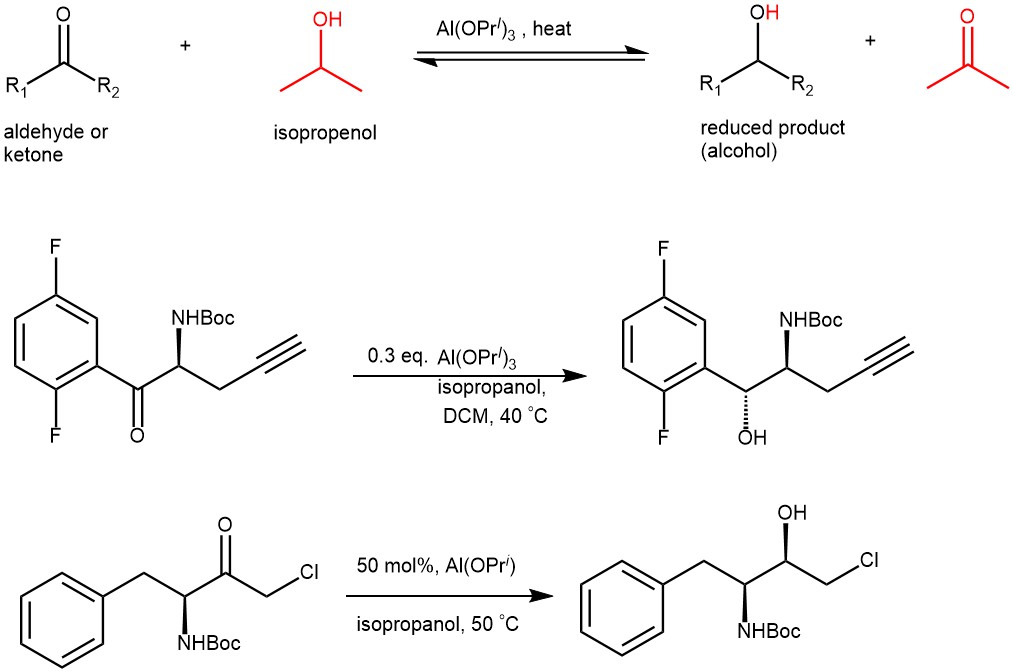
The reduction of aldehydes and ketones to the primary and secondary alcohols represents important synthetic transformations. One of the most chemoselective and mild reactions for these types of reductions is the Meerwein-Ponndorf-Verley (MPV) reductions. It is the reduction of aldehydes and ketones to the corresponding alcohols using aluminum alkoxide (usually aluminum isopropoxide) in the presence of sacrificial alcohol (mostly isopropyl alcohol).
In 1925, it was found independently by H. Meerwein that an aldehyde can be reduced with ethanol in the presence of aluminum ethoxide. In the same year, A. Verley reduced ketones with aluminum ethoxide as well as aluminum isopropoxide. Similarly, in 1926 W. Ponndorf found that by utilizing aluminum alkoxides of more readily oxidizable secondary alcohols, such as isopropyl alcohol, aldehydes and ketones could be reduced readily.
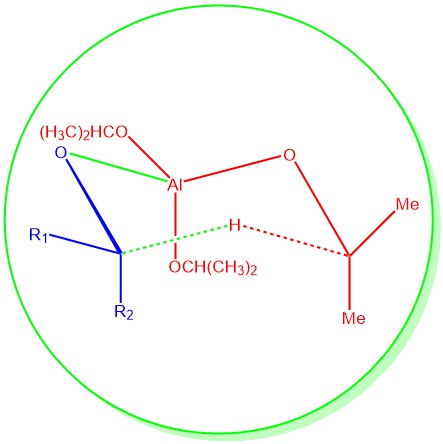
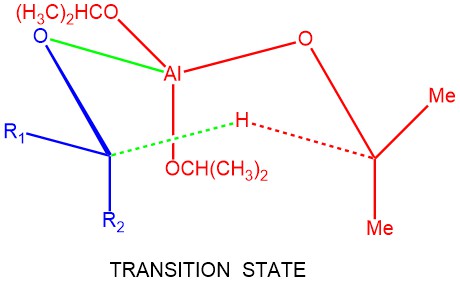
MECHANISM: The Meerwein-Ponndorf-Verley (MPV) reaction involves a hydride transfer, wherein aluminum alkoxide acts as a catalyst. In most cases, the reaction follows a cyclic six-membered transition state, where the reducing agent and the oxidizing agent coordinate to the metal center of the aluminum alkoxide catalyst. After the complexation of the aluminum with the carbonyl oxygen, the critical step is the hydride transfer from the α-position of the isopropoxide ligand to the carbonyl carbon through a six-membered transition state.
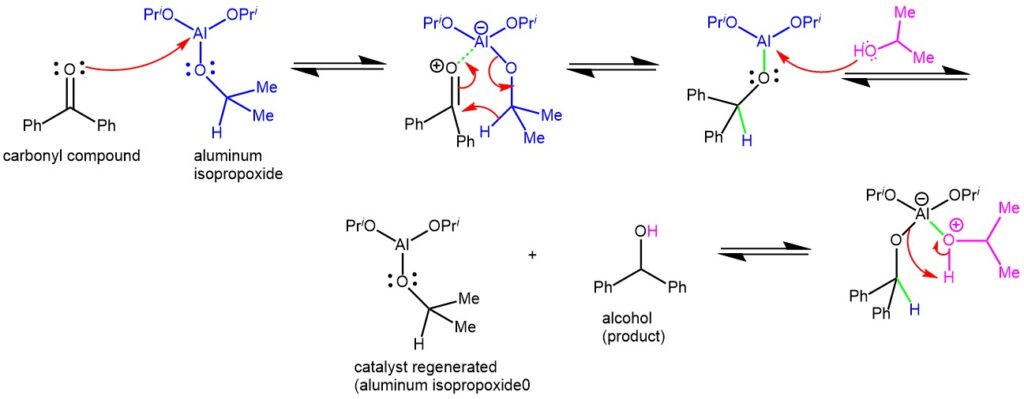
The reaction favors the formation of the most thermodynamically stable product and relies on an excess of the sacrificial hydrogen source (typically isopropanol) while removing acetone. The reaction is known as Oppenauer oxidation when driven in the opposite direction.
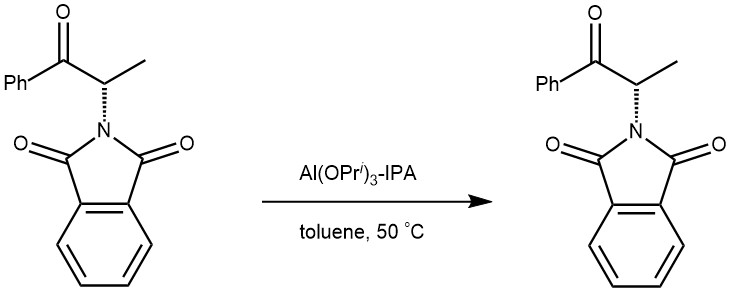
EXAMPLE 1: A mixture of ketone 2a (267 mg, 0.637 mmol, 1.0 equiv), Al(OiPr)3 (26 mg, 0.2 equiv), and iPrOH (0.536 mL, 11 equiv) in toluene (0.80 mL, 1.3 mL/mmol) was heated at 50 °C under N2 for 15 h. The reaction was cooled and quenched with 4 mL of 1 N HCl and 4 mL of EtOAc. The organic layer was washed with 4 mL of water and concentrated. Crude 1H NMR was taken to determine the diaste[1]reoselectivity to be 98.3:1.7. The product was further purified by a hexane tituration to give 260 mg (97% yield) of (1R,2S)-1-(3′,5′-bistrifluoromethylphenyl)-2-benzyloxycarbonylamino-1-propanol (3a) as a white solid with >99% enantiomeric excess (ChiralPak AD-H (4.6 × 150 mm) column, 0.1:10:90 of TFA/iPrOH/heptane, 1.5 mL/min at 30 °C; retention times for desired 1R,2S enantiomers of 2.6min and for undesired, 4.7 min). Mp 141-142 °C.[ REF: J. Org. Chem., Vol. 71, No. 2, 2006]
EXAMPLE 2: A mixture of aluminum isopropoxide (1 mmol) and isopropanol (9.5 mmol) in toluene (2 mL) was added dropwise to the reactant (1 mmol) in toluene (3 mL) at room temperature. The reaction mixture was then stirred at 50 °C overnight. The solution was extracted with ethyl acetate (3 × 5 mL). The combined organic phases were washed with 1M HCl (20 mL) and brine (20 mL) and dried over anhydrous Na2SO4.The solution was filtered, and the solvent was evaporated to give a yellow viscous oil, which was chromatographed with ethyl acetate and petroleum (V/V = 1:4) to give the product. [REF: Journal of Chemical Research, 2016, 40, 82-86]
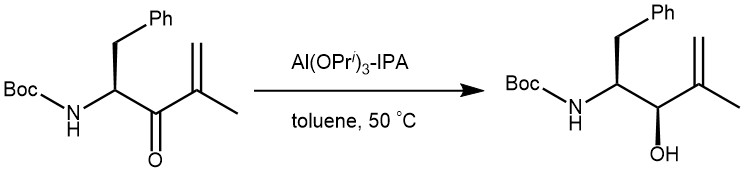
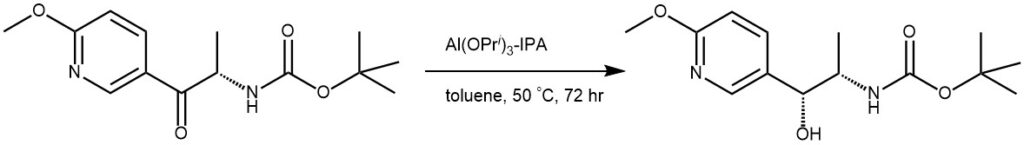
EXAMPLE 3: To the starting material (102 g, 364 mmol) in 2-propanol (310 mL) and toluene (475 mL) was added aluminum tri iso-propylate (44.6 g, 218 mmol). The reaction mixture was stirred at 50 °C for 72 hours and water (220 mL) and ethyl acetate (550 mL) were added. The mixtures pH is adjusted to 4 (10 mL 6M HCl) and extracted with ethyl acetate. The organic phase is washed with brine (220 mL), dried over MgSO4, the solvent is removed and the product purified by chromatography on silica gel (3000 g, ethyl acetate in hexane 0% to 50%). Yield 100.7 g (98%).[REF: World Intellectual Property Organization WO 2009/142569 A1]