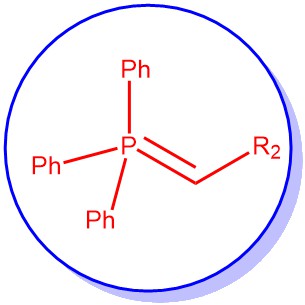
The Wittig reaction is perhaps the most commonly used method for the synthesis of alkenes. The reaction occurs between a carbonyl compound (aldehyde or ketone) and a phosphorus ylide to give an alkene with phosphorus oxide as the by-product. In other words, the formation of carbon-carbon double bond (olefins/alkenes) from carbonyl compounds (aldehyde/ketone) and phosphorus ylides (phosphoranes) is known as the Wittig reaction. It is regiospecific: alkene is invariably formed from the ylide α-carbon to the carbonyl carbon.
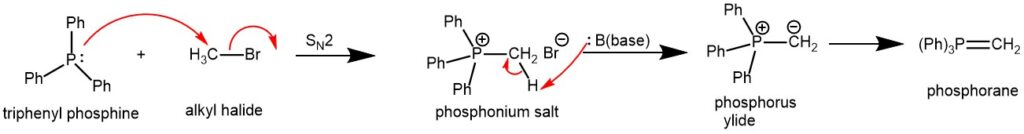
The ylides are accessible by deprotonation of the parent phosphonium salt. The phosphonium salt in turn is obtained from alkyl halide and triphenylphosphine.
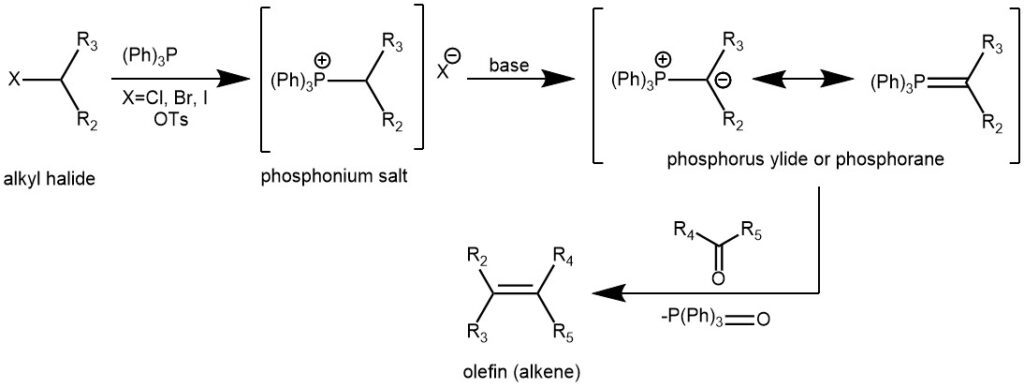
PHOSPHONIUM YLIDES (PHOSPHORANES):
The phosphorus ylide is usually prepared from a trialkylphosphine or triphenylphosphine and an alkyl halide (1° or 2°) followed by deprotonation with a suitable base (e.g., RLi, NaH, NaOR, NaOH, LiHMDS, LDA, etc).
Phosphorus ylides are broadly categorized according to the nature of the substituents attached to the α-carbon (R2 in the figure).
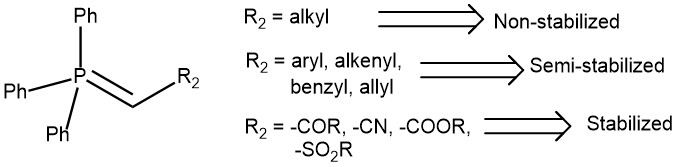
- If R2 is an alkyl group, the ylide is “non-stabilized” or reactive and are water or air sensitive
- If R2 is phenyl, alkenyl, benzyl, and allyl, it is “semi-stabilized” and less prone to hydrolysis.
- If R2 is carbonyl, ester, sulfone, or cyanide, the ylide is “stabilized”
The nature of the ylide used in the Wittig reaction dictates the stereoselectivity of the reaction.
- Non-stabilized ylides under salt-free conditions afford high (Z)-selectivity with aldehydes.
- Stabilized ylides give predominantly (E)-olefins with aldehydes under salt-free conditions.
- Semi-stabilized ylides usually give alkenes with poor stereoselectivity.
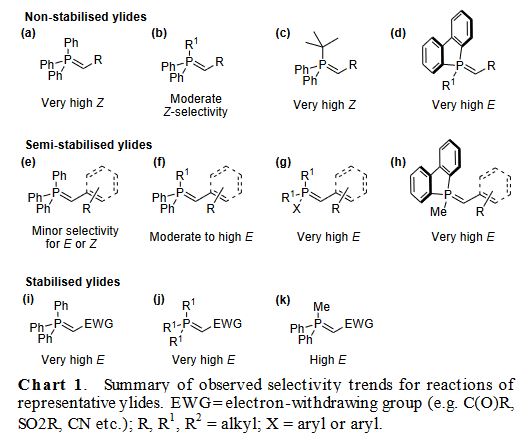
MECHANISM: The nucleophilic attack by the ylide on the carbonyl function (like in an aldol reaction) forms a betain intermediate. The intermediate can form a covalent four-membered ring structure (oxaphosphetane). Subsequent decomposition of the oxaphosphetane produces the olefin and the phosphine oxide as the by-product. The driving force for this reaction is the formation of the very stable P=O bond (in phosphine oxide).
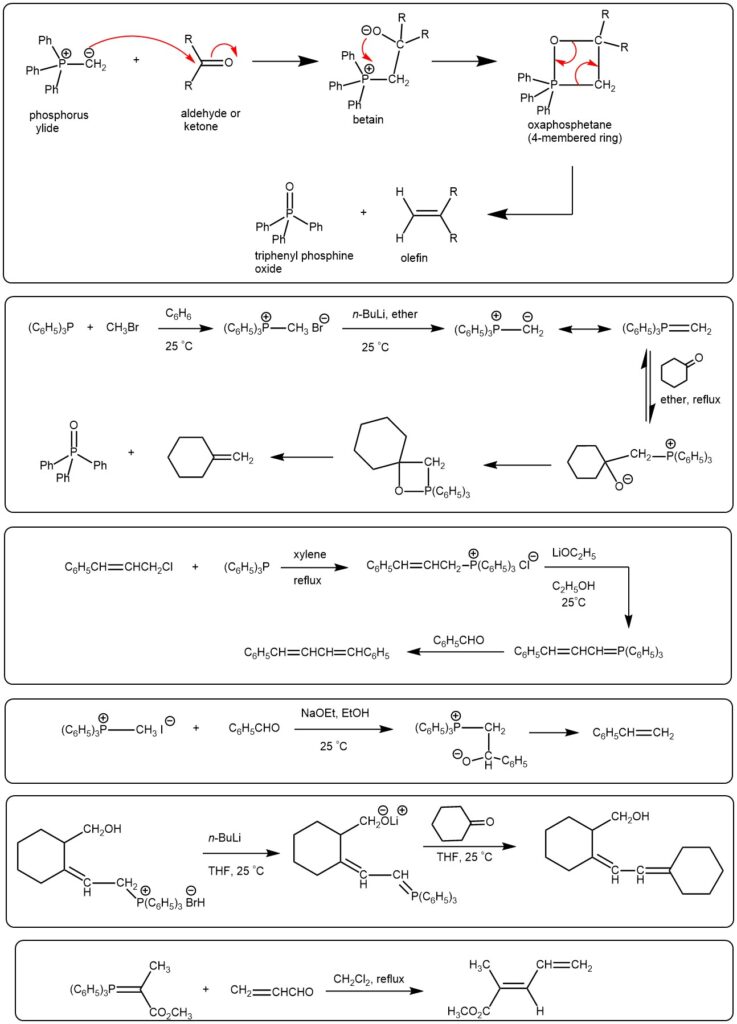
EXAMPLE 1: To a solution of the aldehyde (25.0 g, 113.1 mmol) in EtOAc (113 mL) was added PPh3 (41.49 g, 158.4 mmol), sat aq NaHCO3 (226 mL), and ethyl 2-bromoacetate (28.34 g, 169.7 mmol). The reaction mixture was stirred vigorously at RT for 3 h. The mixture was diluted with H2O (150 mL) and EtOAc (300 mL). The organic layer was washed with H2O (250 mL), brine (250 mL), dried (Na2SO4), and concentrated. The resulting residue was purified by silica gel column chromatography (40:1 PE/EtOAc) to provide the product as a white solid. [33.25 g, 100%]. [REF: World Intellectual Property Organization WO2016011930]
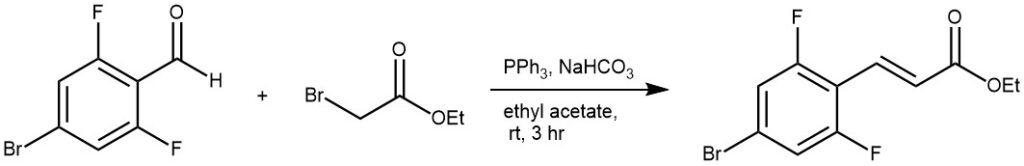
EXAMPLE 2: Potassium tert-butoxide (2.60 g, 23.2 mmol) was added to a solution of 11.6 g (28.6 mmol) triphenylmethyl phosphonium iodide in 50 mL of dry THF at 0 °C. After the mixture was stirred for 15 min at room temperature, 6.50 g (24.0 mmol ) of 4-(N,N-diphenylamino)benzaldehyde was added at 0 °C and the mixture was stirred at room temperature for another 3 hour. It was then poured into 400 mL water. The solid was collected by filtration. The crude was recrystallized in ethyl alcohol to give a white solid in a yield of 89%.[REF: Tetrahedron, 2011, 67, 44, 8477-8483]
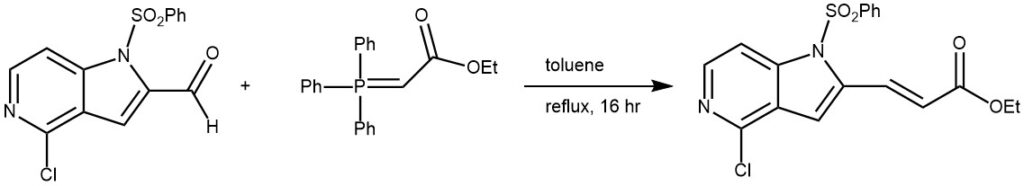
Example 3: A mixture of the aldehyde (1.0 g, 3.11 mmol) and (carbethoxymethylene)triphenylphosphorane (2.11 g, 6.22 mmol) in toluene (30 mL) was refluxed for 16 h. The solvent was removed in vacuo and the resulting residue was purified by silica gel column chromatography to provide the product as a white solid. [1.05 g, 84%].[REF: World Intellectual Property Organization WO2015088045]
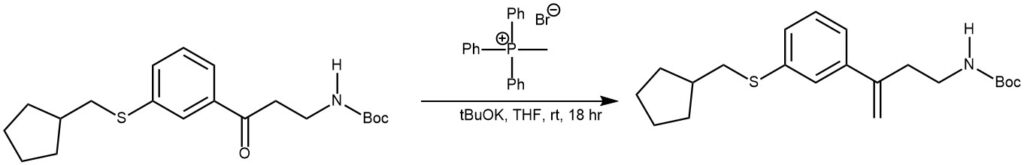
EXAMPLE 4: To a suspension of methyl triphenylphosphonium bromide (2.4 g, 6.6 mmol) in THF was added tBuOK (1M in THF, 7.1 mmol) at RT. After stirring for 90 min, a solution of the SM (1.25 g, 3.35 mmol) in dry THF was added. The resulting mixture was stirred at RT for 18 h, after which time it was partitioned between saturated NH4Cl and MTBE. The org layer was dried (Na2SO4), concentrated, and purified by flash chromatography (25-30% EtOAc/hexane) to provide the product as a colorless oil. [0.3 g, 24%] [UK Pat App GB2463151A, page 141]
REFERENCES:
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako
- Modern synthetic reactions by Herbert O. House
- Byrne, Peter A., and Declan G. Gilheany. “The Modern Interpretation of the Wittig Reaction Mechanism” 42, no. 16 (May, 2013) DOI: 10.1039/c0xx00000x
- https://chem.libretexts.org/