The introduction of formyl (-CHO) group into electron -rich aromatic compounds using a Vilsmeier reagent is known as the Vilsmeier-Haack formylation reaction. The Vilsmeier reagent is prepared from any N, N-disubstituted formamide by reacting it with an acid chloride like phosphorus oxychloride (POCl3), oxalyl chloride ((COCl)2), or thionyl chloride (SOCl2). Most oftenly, the combination of dimethylformamide (DMF), and phosphorus oxychloride (POCl3) is used.
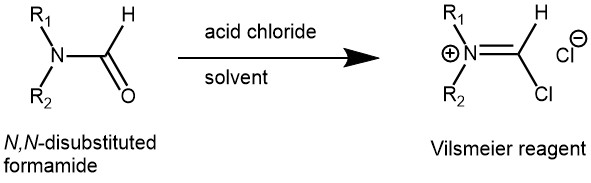
MECHANISM OF VILSMEIER REAGENT PREPARATION USING PHOSPHORUS OXYCHLORIDE (POCl3)
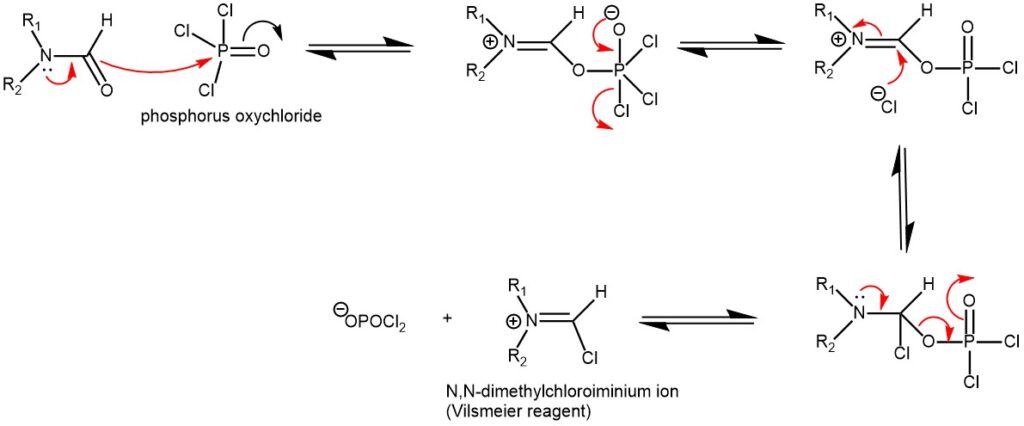
MECHANISM OF VILSMEIER REAGENT PREPARATION USING OXALYL CHLORIDE (COCl)2
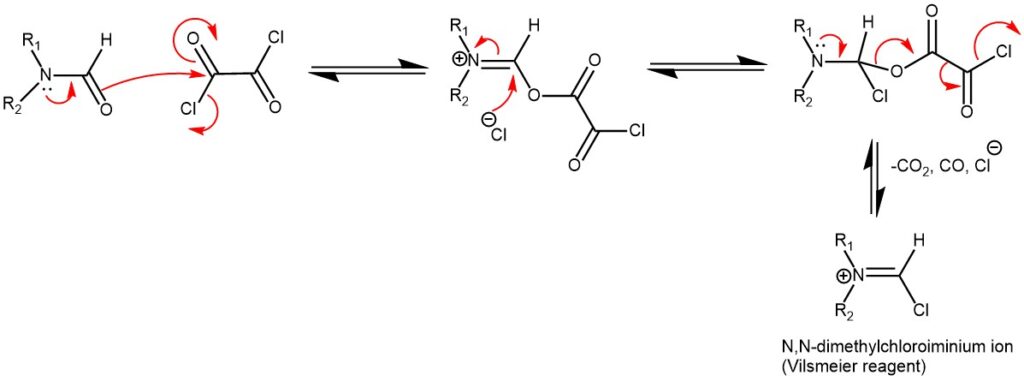
MECHANISM OF VILSMEIER REAGENT PREPARATION USING THIONYL CHLORIDE (SOCl2)
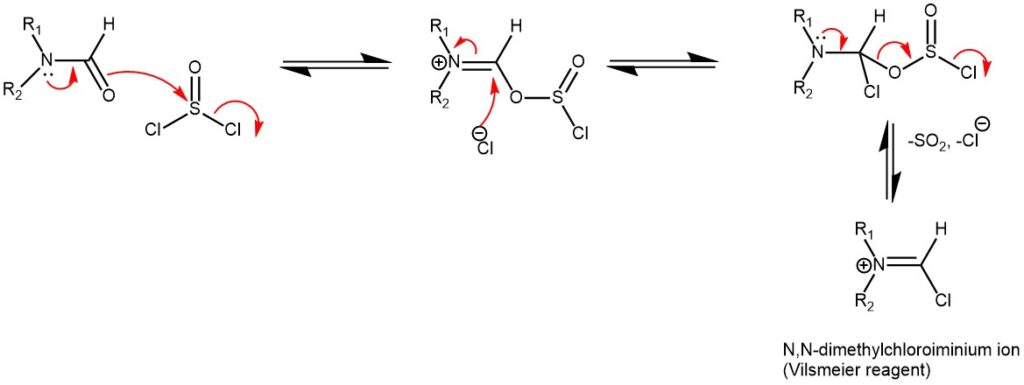
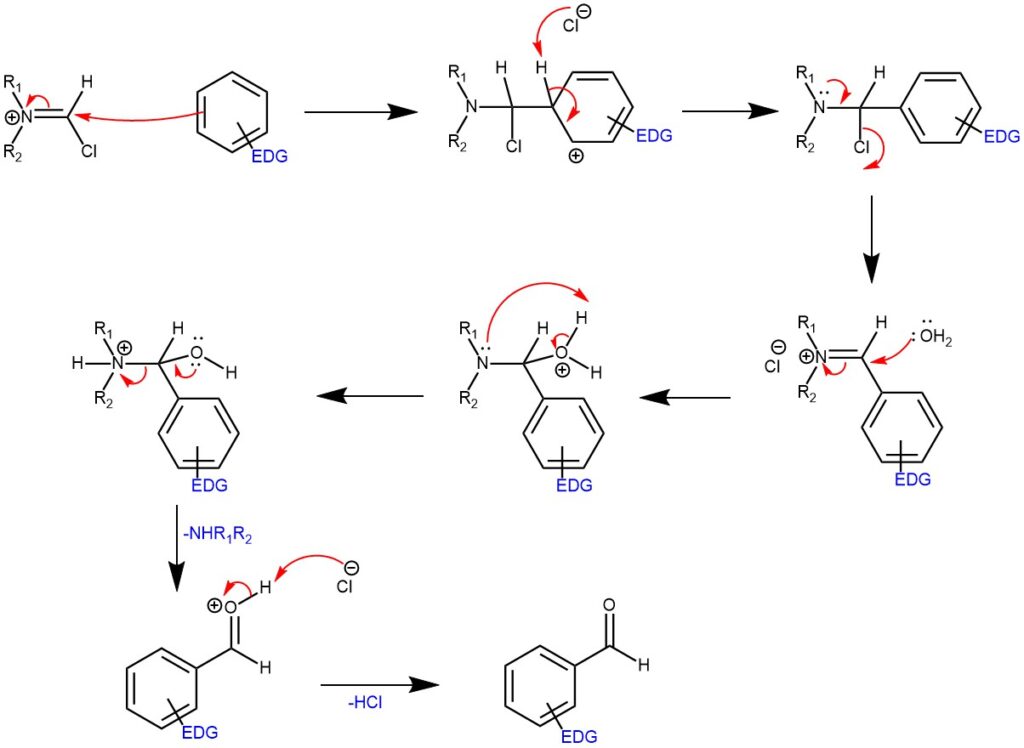
MECHANISM OF VILSMEIER-HAACK FORMYLATION USING VILSMEIER REAGENT:
The Vilsmeier-Haack Reaction is often a reliable method to install a formyl group on aromatic moieties. The active reactant and formylating agent N,N-dimethylchloroiminium ion (Vilsmeier reagent) is also commercially available, and it can be generated in situ by the reaction between phosphoryl chloride and DMF or a similar formamide. Oxalyl chloride or thionyl chloride can also be used to generate the active ion. Upon reacting with an aromatic ring, the resulting product furnishes an iminium ion, which is then rapidly converted to the aldehyde during aqueous workup. Like most aromatic substitution reactions, Vilsmeier-Haack reaction tends to operate more efficient on electronically rich aromatic rings, and typically add to the para position or most electron-rich carbon.
Examples:
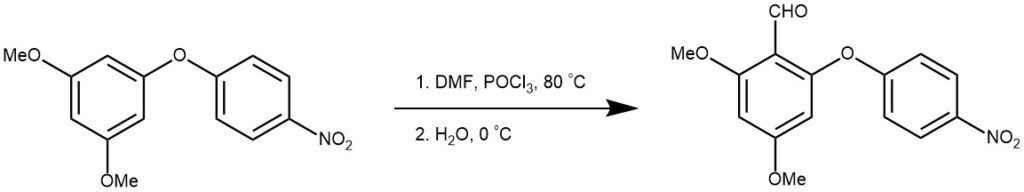
Phosphoryl chloride (5.0 ml, 55.0 mmol) was carefully added to ice-cold DMF (4.2 ml, 55.0 mmol) at 0 C. A solution of 1, 3-dimethoxy-5-(4-nitrophenoxy) benzene 5 (3.0 g, 11.0 mmol) in DMF (10 ml) was added and the resulting mixture was heated at 80 C for 3 h. The dark viscous mixture was then poured into ice-cold water (50 ml) and stirred vigorously for 2 h to coagulate any precipitate, which was filtered off. The precipitate was washed with water (50 ml), dried and chromatographed [DCM/EtOAc, 99:1] to yield the title compound.
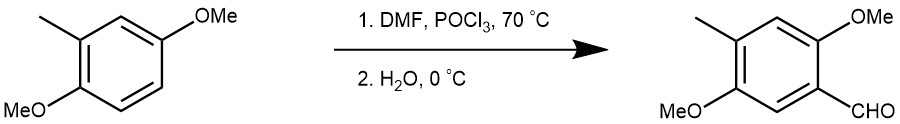
A mixture of POCl3 (13.4 mL, 144 mmol) and N-methylformanilide (15.3 mL, 124 mmol) was stirred at rt for 50 min. 2,5-dimethoxytoluene 8 (1.56 g, 33.9 mmol) was added all at once and the solution was warmed to 70 °C. After the mixture was stirred for 1 h, the hot reaction mixture was poured into ice water and extract with AcOEt. The combined extracts were washed with saturated aqueous NaCl. The residue upon workup was chromatographed on silica gel with hexane−AcOEt (9:1 v/v) as eluent to give benzaldehyde
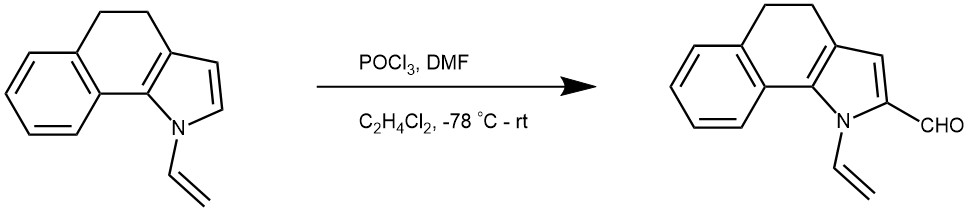
To DMF (0.40 g, 5.5 mmol) at -78 °C (acetone-dry ice bath), POCl3 (0.84 g, 5.5 mmol) was added dropwise, and the mixture obtained was stirred for 15 min without cooling. Then 1,2-dichloroethane (1.5 mL) was added, and after cooling to -78 °C, a solution of N-vinylpyrrole (5 mmol) in 1,2-dichloroethane (1.5 mL) was added dropwise over 10 min. The resulting mixture was stirred for 3 h at ambient temperature. Next, a solution of sodium acetate (2.26 g, 28 mmol) in water (10 mL) was added and stirring was continued for 1 h at ambient temperature. The reaction mixture was extracted with diethyl ether (5 · 5 mL), the combined organic phases were washed with saturated aqueous sodium hydrocarbonate solution (3 · 5 mL) and dried over magnesium sulfate. The residue obtained after evaporation of the ether was purified on basic alumina (hexane/ether 3:1) to give formylpyrrole.
More examples:
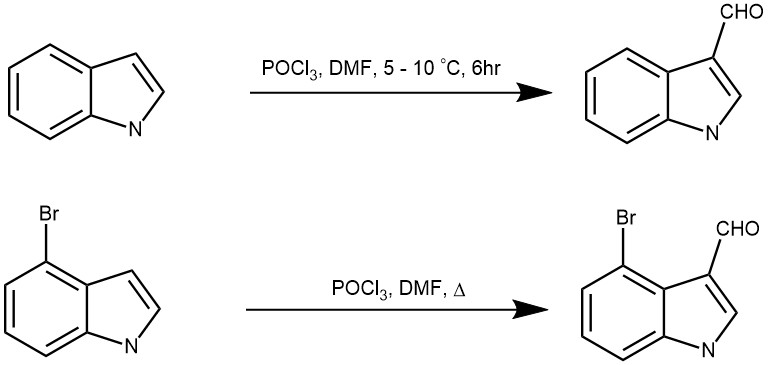
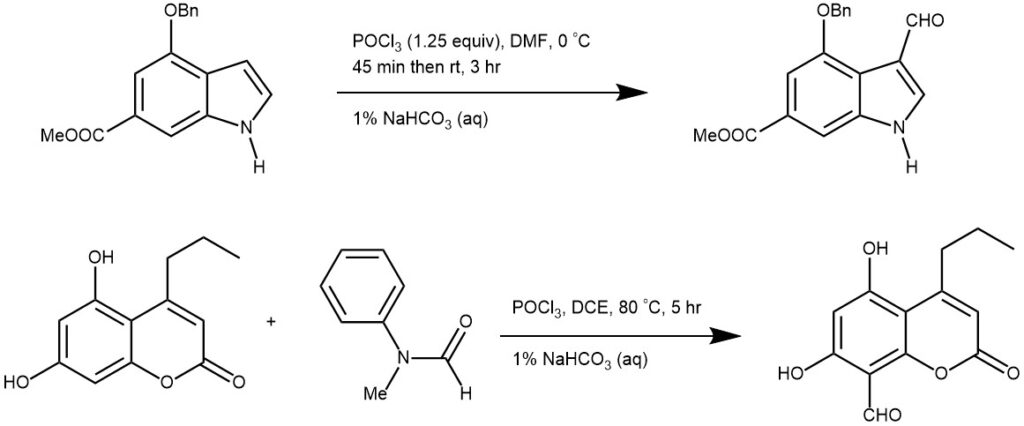
The Vilsmeier-Haack formylation reaction is a versatile and powerful method for the synthesis of aldehydes from aromatic compounds. The reaction proceeds through the use of the Vilsmeier reagent, which is easily prepared from DMF and POCl3. The reaction has numerous synthetic applications, including the synthesis of heterocyclic compounds, and is also a useful tool in analytical chemistry.



