Transesterification:
Transesterification reaction, also called alcoholysis, is the displacement of alcohol from an ester by another alcohol. In other words, it is an organic transformation converting one ester into another.
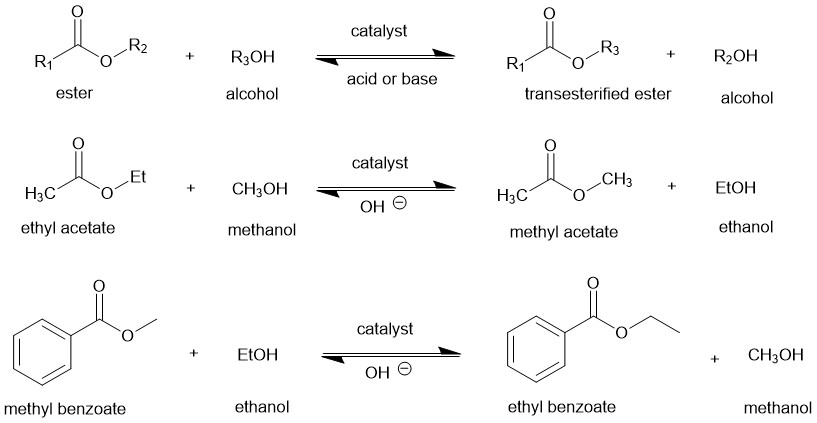
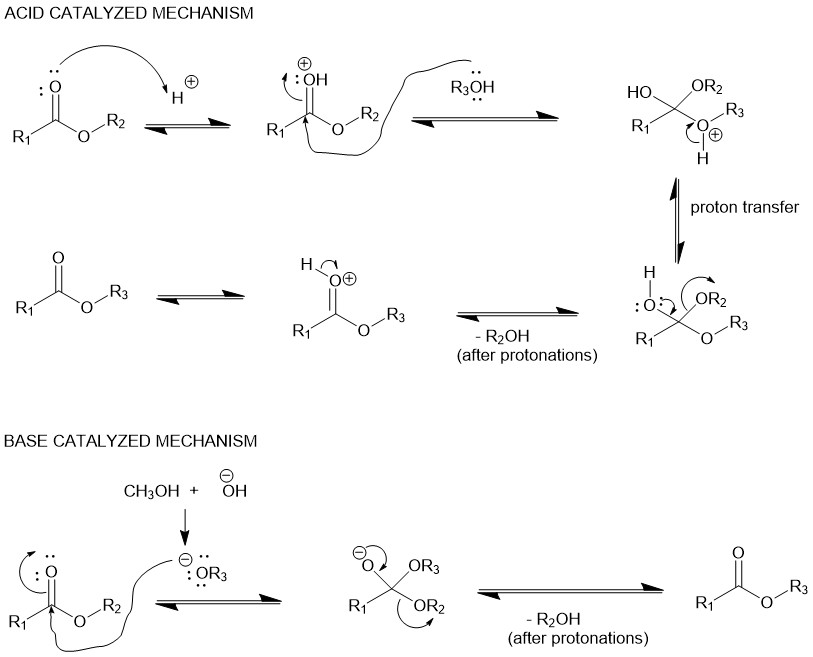
Transesterification is catalyzed by acids or bases. It is an equilibrium reaction and shifts in the forward direction by using an excess of alcohol (reactant) or removing one of the products as it is formed.
Biodiesel:
Diesel fuel is a mixture of hydrocarbons that typically contain between 9 and 25 carbon atoms per molecule. Diesel fuels have an essential function in the industrial economy of a country as they are used in combustion engines. This petroleum diesel or petrodiesel is obtained by the fractional distillation of crude oil.
On the other hand, biodiesel is a renewable, clean-burning liquid fuel obtained from vegetable oil, fats, and waste cooking oils. Chemically, biodiesel is a mixture of fatty acid alkyl esters derived from triglycerides. Just as a reminder, fats and oils are called triglycerides as they are composed of three fatty acid molecules joined to glycerol.
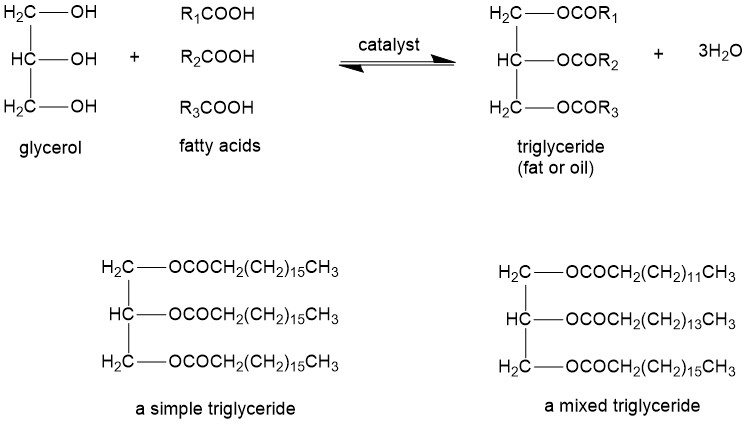
R1, R2, and R3 are the portions from long-chain fatty acid and can be all same ( it is then called simple triglyceride) or different ( it is then called mixed triglyceride). Simple triglycerides are very uncommon. A triglyceride is called fat if it is a solid at room temperature or it is an oil. Some common fatty acids found in fats and oils are lauric acid, myristic acid, palmitic acid, stearic acid, oleic acid, linoleic acid, linolenic acid, etc.
Biodiesel is a nonpetroleum-based fuel that generally consists of fatty acid alkyl esters. Thes fatty acid alkyl esters, called biodiesel, are obtained by transesterification of the triglyceride. The transesterification reaction converts the triglyceride into smaller esters whose size and properties are similar to those of petroleum diesel. The overall transesterification reaction of the triglyceride can be represented as:
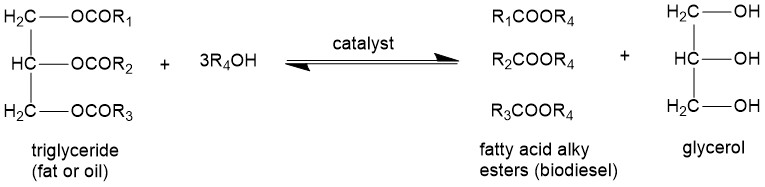
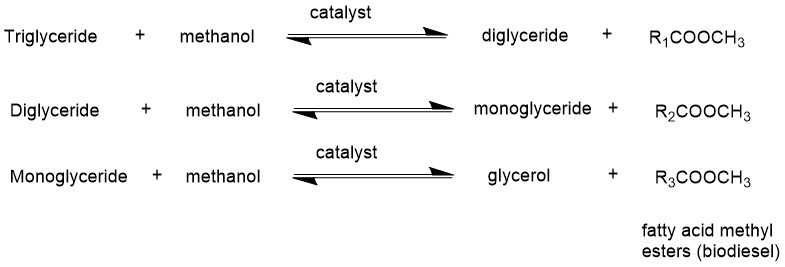
Glycerine (glycerol) is formed as a byproduct. Stoichiometrically, three moles of alcohol are required per mole of triglyceride. However, a higher ratio of alcohol is used to push the equilibrium in a forward direction. Alcohols used most commonly are methanol and ethanol. Technically, transesterification consists of three consecutive reversible reactions. Alkali or alkaline alkoxides (eg. Sodium methoxide) and hydroxides (eg. KOH, NaOH) are the most efficient catalysts because these require short reaction times and low temperatures than the acid catalysts. Biodiesel production by transesterification consists of a sequence of three consecutive reversible reactions. The first step is the conversion of triglycerides to diglycerides, followed by the conversion of diglycerides to monoglycerides, and finally monoglycerides into glycerine, yielding one ester molecule from each glyceride at each step.
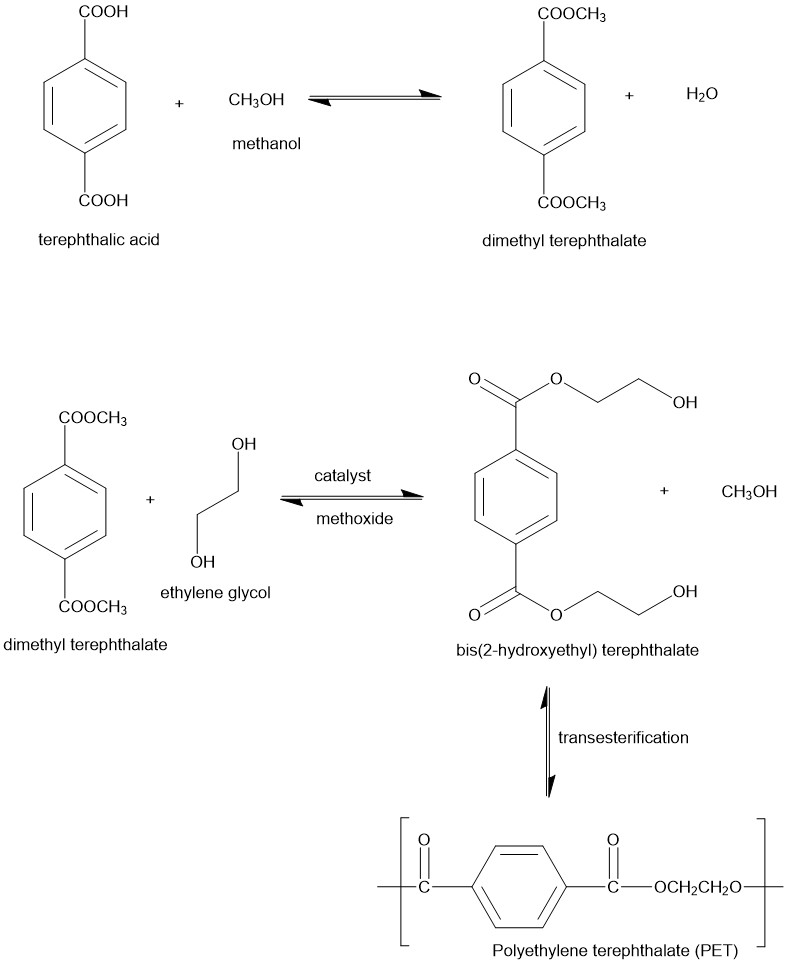
Polyethylene Terephthalate (PET):
Polyesters are polymers obtained by the condensation reaction of a dicarboxylic acid and a diol characterized by the presence of ester functions (-COO-) along the chain. PET is an aliphatic polyester used in everyday items like packaging, textiles, etc. It is derived from a combination of two monomers: dimethyl terephthalate (DMT) and ethylene glycol (EG) employing a transesterification reaction. Dimethyl terephthalate is obtained by esterification of terephthalic acid with methanol. The dimethyl terephthalate then undergoes transesterification with ethylene glycol to form bis (2-hydroxyethyl)terephthalate at temperatures of 150 – 210 °C and the typical catalysts used are metal derived and based on titanium and antinomy. Bis (2-hydroxyethyl)terephthalate is then heated between 250 °C and 300 °C under a vacuum to form the polyester by transesterification.