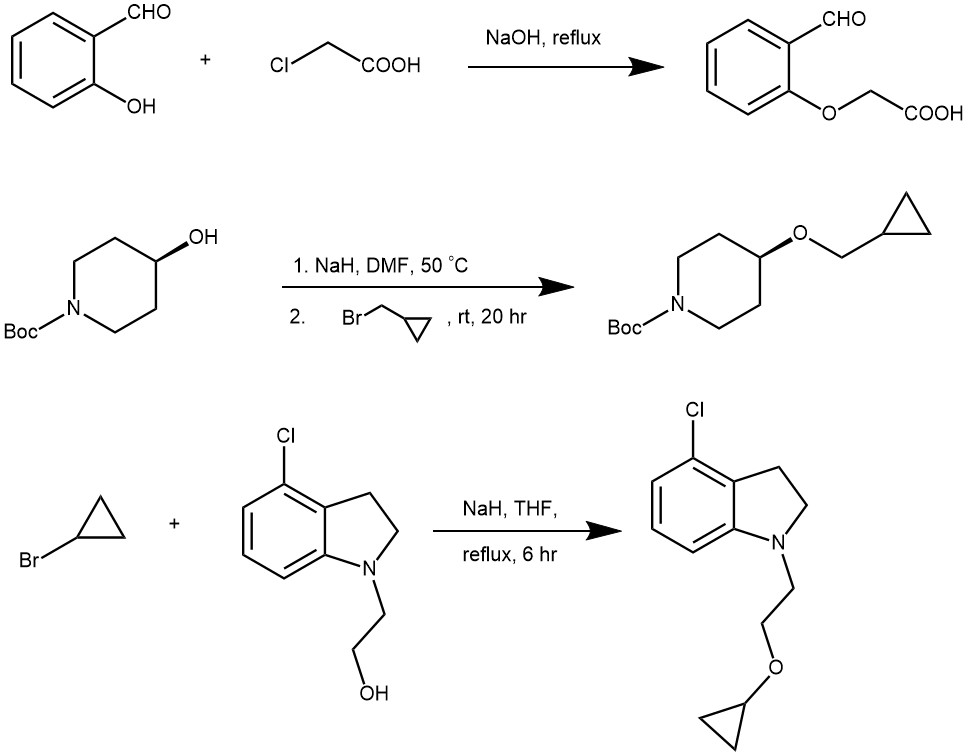
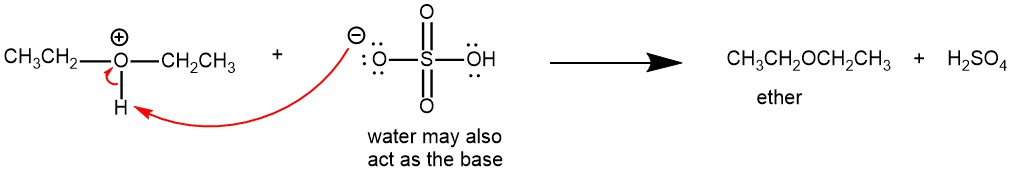
ACID–CATALYZED DEHYDRATION OF ALCOHOLS
Diethyl ether and several other symmetrical ethers are prepared by the acid-catalyzed dehydration of alcohols.

The yields are usually higher for symmetrical ethers formed from unbranched primary alcohols. The yields are lower for the secondary alcohols and tertiary alcohols give alkene as the major product instead of ethers.
MECHANISM
The proton transfer from the acid to the alcohol gives an oxonium ion, which converts -OH to a good leaving group (H2O).
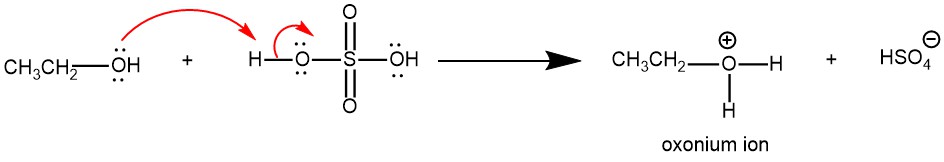
Nucleophilic displacement of water (H2O) by the -OH group of a second alcohol molecule gives a new oxonium ion.
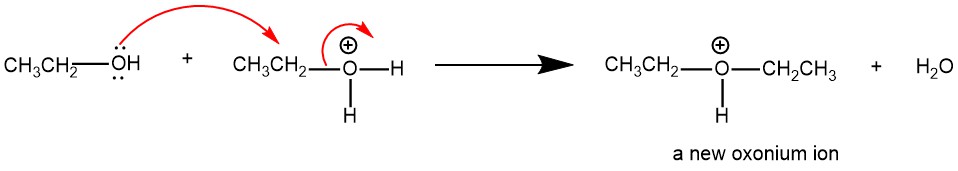
Proton transfer from the new oxonium ion to water (H2O) or bisulfate (HSO4–) ion completes the reaction.
THE OXYMERCURATION-DEMERCURATION:
The oxymercuration-demercuration reaction is a method for the synthesis of ethers from alkenes. The reaction proceeds via the addition of mercuric acetate (Hg(OAc)2) or mercuric trifluoroacetate (Hg(OCOCF3)2) to the alkene, followed by reduction of the intermediate mercurinium ion with a reducing agent such as sodium borohydride (NaBH4) in presence of sodium hydroxide (NaOH). The intermediate product is an alkylmercury compound, which can react with an alcohol to form the corresponding ether.
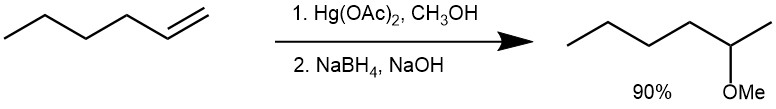
In a typical experiment, mercuric acetate (1 equivalent) is added to a flask containing the alcohol followed by the addition of the alkene (1 equivalent) and stirred vigorously. After a while (~10 minutes), the reduction of the mercurial intermediate is achieved by adding 3M sodium hydroxide and an equal volume of 0.5 M sodium borohydride in 3M sodium hydroxide in water. The mixture is allowed to stir for 2 hours, until the mercury is coagulated and settled.
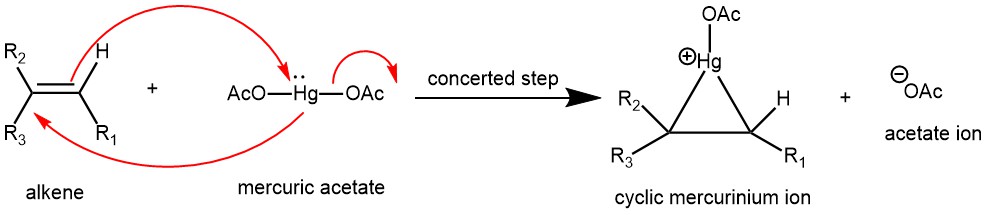
MECHANISM
This reaction obeys the electrophilic addition mechanism. The main difference is that the carbocation intermediate is stabilized by a mercurium ion bridge, which prevents it from rearranging. Metals have an electropositive charge. The electrophile in the acetate complex is mercury, which has a partial positive charge.
The Nucleophilic C=C bond attacks the electrophilic mercury (Hg) while the acetate ion leaves as the leaving group, forming cyclic mercurinium ion.
To open the mercurinium ion bridge, an alcohol molecule attacks the most substituted carbon.
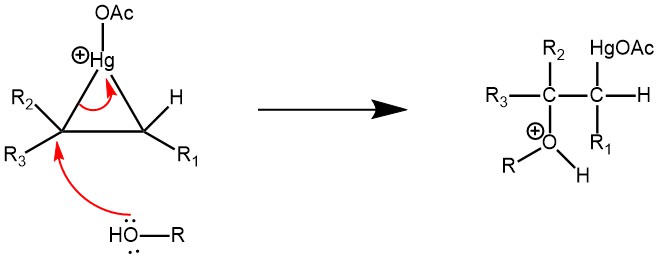
Deprotonation of the oxonium ion by the acetate ion (acting as a base) is the next step.
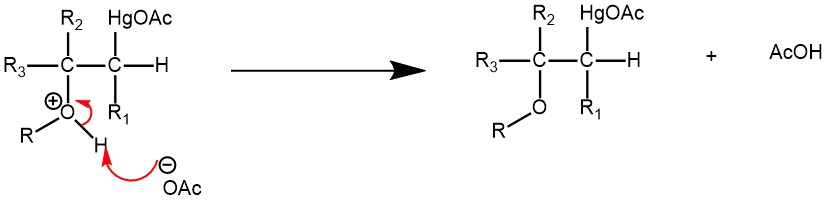
Treatment with sodium borohydride replaces the acetoxy mercury with a hydride giving the final ether product.
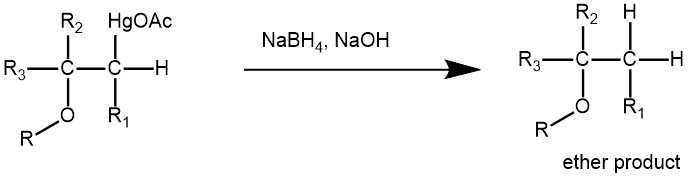
The reaction mechanism is based on Markovnikov’s regioselectivity, with the OR group attached to the most substituted carbon and the H group attached to the least substituted carbon. The reaction is useful since it does not require strong acids and prevents carbocation rearrangements because no separate carbocation intermediate forms.
The oxymercuration-demercuration reaction has several advantages over other methods for ether synthesis. It is a stereospecific reaction that proceeds with Markovnikov regioselectivity. Additionally, the reaction is less likely to form byproducts or rearrangement products, unlike other methods. One limitation of the oxymercuration-demercuration reaction is that it requires the use of toxic and expensive mercuric acetate, which can be a hazard to handle and dispose of. As a result, other methods such as the Williamson ether synthesis or acid-catalyzed dehydration of alcohols are often preferred for large-scale synthesis.
MITSUNOBU REACTION
The substitution of primary and secondary alcohols with a nucleophile in the presence of dialkyl azodicarboxylate [ eg. Diethyl azodicarboxylate (DEAD)] and a trialkyl or triaryl phosphine [eg. Triphenylphosphine (PPh3)] is known as the Mitsunobu reaction. The primary and secondary alcohols are best suited for this reaction. When secondary alcohol is used there is an inversion of configuration. The nucleophiles are relatively acidic compounds (pKa < 15) since the reagent (DEAD) must be protonated during the reaction to prevent side reactions. When phenol is used as a nucleophile, ethers are the product. The Mitsunobu reaction of phenol and aliphatic alcohols is considered an essential reaction for aryl ether formation.
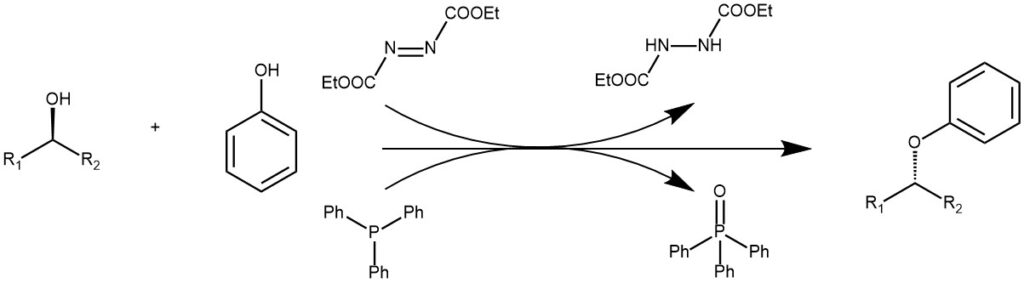
In a typical experiment, the alcohol (1 equivalent) is added to a solution of phenol (1.1 equivalent) and triphenylphosphine (1.1 equivalent) in anhydrous THF under N2 at 0 °C. The resulting suspension/solution was treated with DEAD (or DIAD)(1.1 equivalent) and the reaction mixture is continued to stir at room temperature until completion.
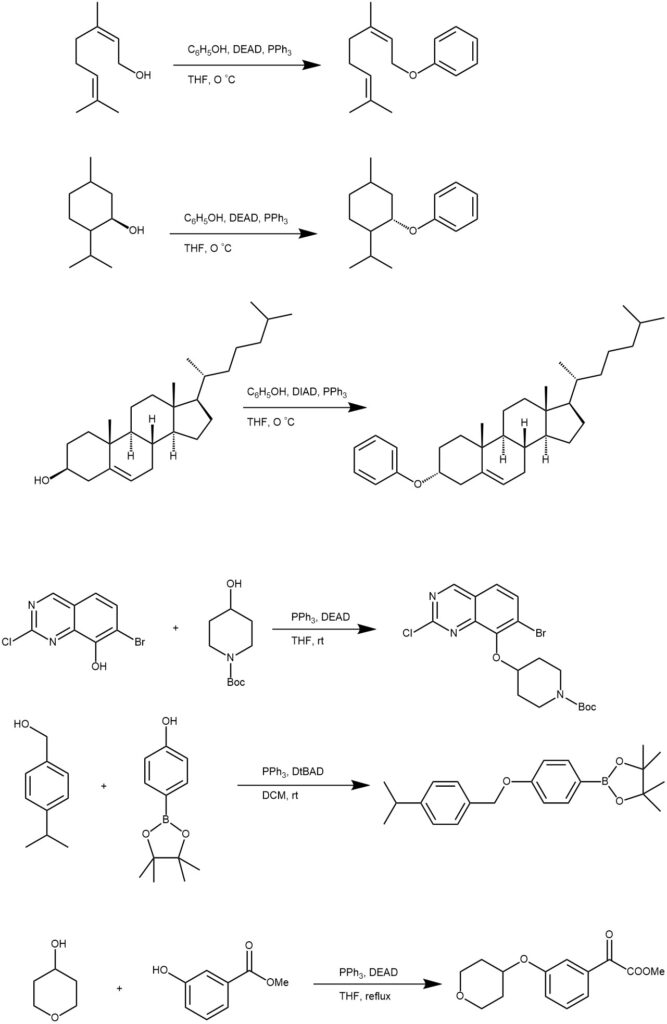
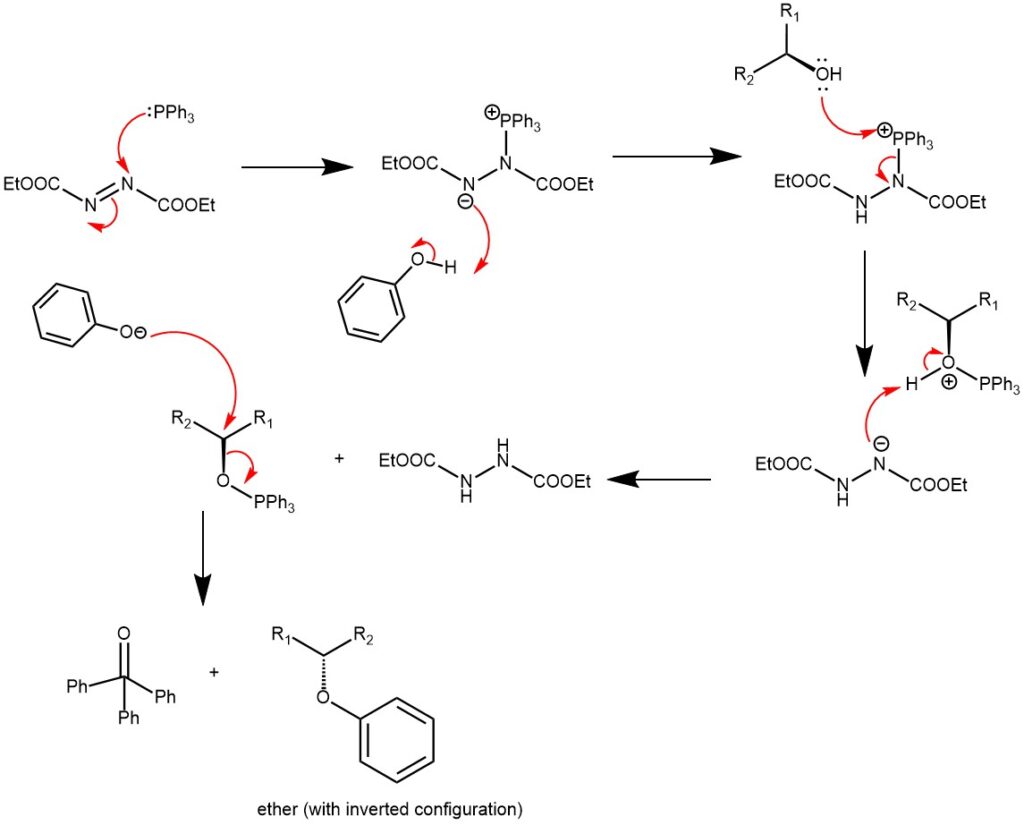
MECHANISM:
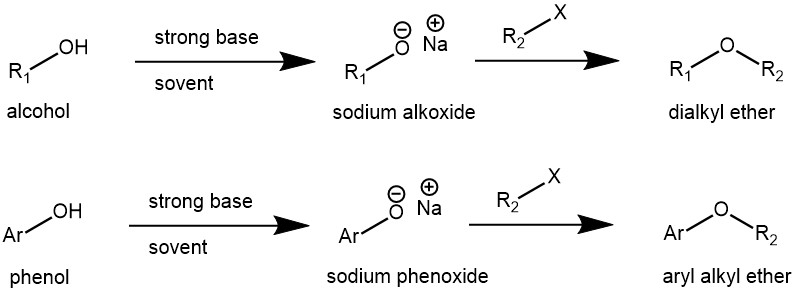
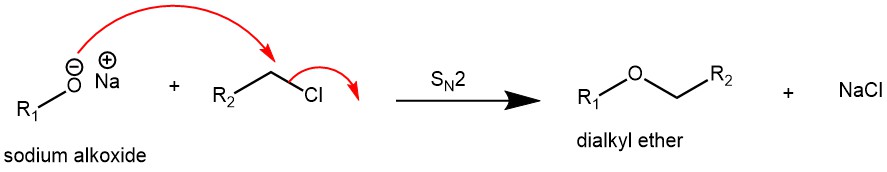
WILLIAMSON ETHER SYNTHESIS
The reaction of aliphatic or aromatic alkoxides with alkyl, allyl, or benzyl halides to afford the corresponding ethers is known as the Williamson ether synthesis. The alkoxides of simple aliphatic primary, secondary, and tertiary alcohols can be easily prepared by the use of strong bases like sodium hydride (NaH), potassium hydride (KH), LHMDS, LDA, etc. while phenoxide can be obtained from weak bases like sodium hydroxide (NaOH), potassium hydroxide (KOH), potassium carbonate (K2CO3), cesium carbonate (Cs2CO3), etc.
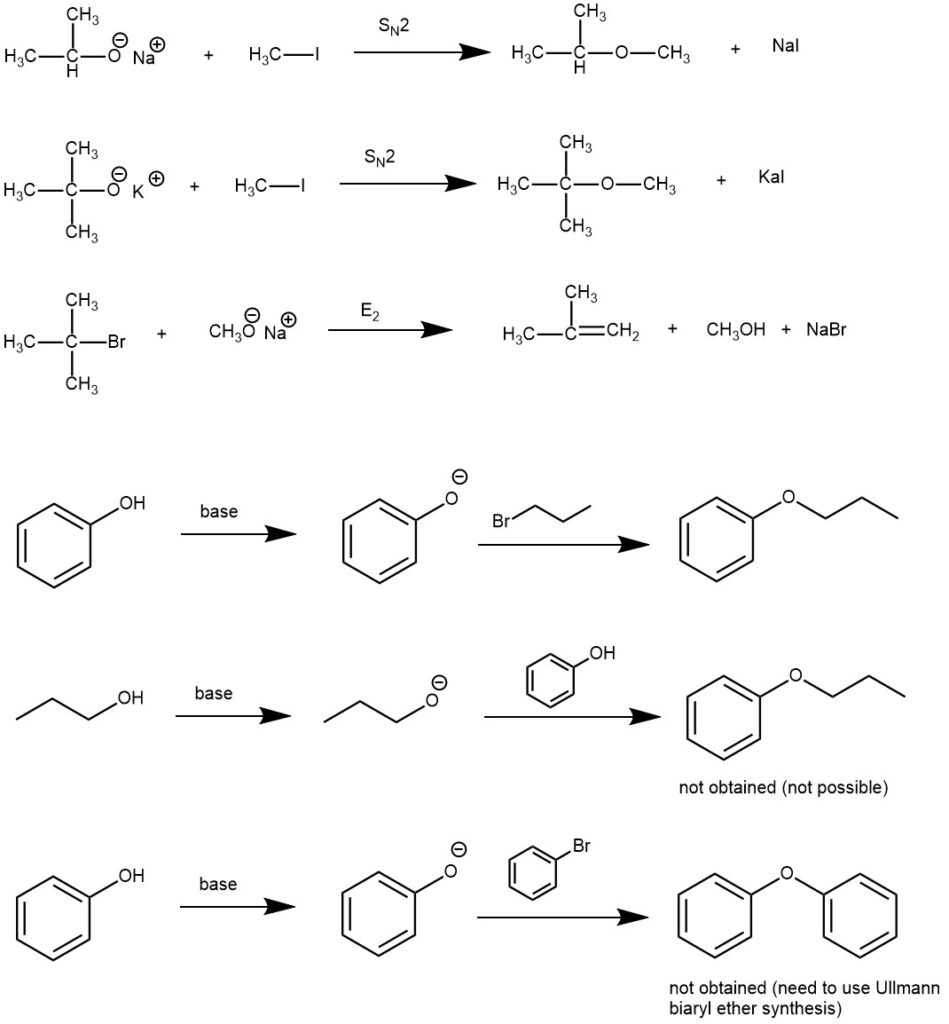
The choice of the alkyl halide component is critical to the success of the reaction: primary, methyl, allylic, or benzylic halides give the highest yields since these undergo SN2-type halide displacement by the alkoxide nucleophile. Tert-butyl methyl ether can be prepared by the reaction of potassium tert-butoxide and iodomethane. On the other hand, the alternative combination of sodium methoxide and 2-bromo-2-methylpropane would give an alkene as the major product by competing elimination reaction. To prepare the aryl alkyl ethers, the aryl component should come from the phenol (phenoxide) and not the halide. The preparation of diaryl ethers from penoxides and unactivated aryl halides is not possible under the conditions of Williamson ether synthesis.
MECHANISM:
Williamson ether synthesis proceeds via an SN2 mechanism.
In a typical experiment, to the solution of the alcohol in DMF, sodium hydride is added in portions under an inert atmosphere at 0 °C and stirred for a while. The halide is then added and stirred again until the reaction is completed.