The Reimer Tiemann reaction was first reported by Karl Reimer in 1876, who obtained salicylaldehyde from phenol and chloroform in an alkaline solution. He also observed that other phenols gave similar products but with varying yields and selectivities. In 1881, Ferdinand Tiemann joined Reimer’s research group and improved the reaction conditions by using sodium ethoxide instead of sodium hydroxide as the base. He also extended the scope of the reaction to other aromatic compounds, such as naphthols, pyrroles, indoles, hydroxyquinolines, hydroxy pyrimidines, and anilines. Together, they published several papers on the Reimer Tiemann reaction and its applications.
It is a method (reaction) for the preparation of phenolic aldehydes by the action of chloroform (CHCl3) on phenols in an alkaline medium. The substitution occurs generally ortho– as well as para to the phenolic group. However, the ortho: para ratio appears to depend upon the solvent and the substituents present on the aromatic ring.
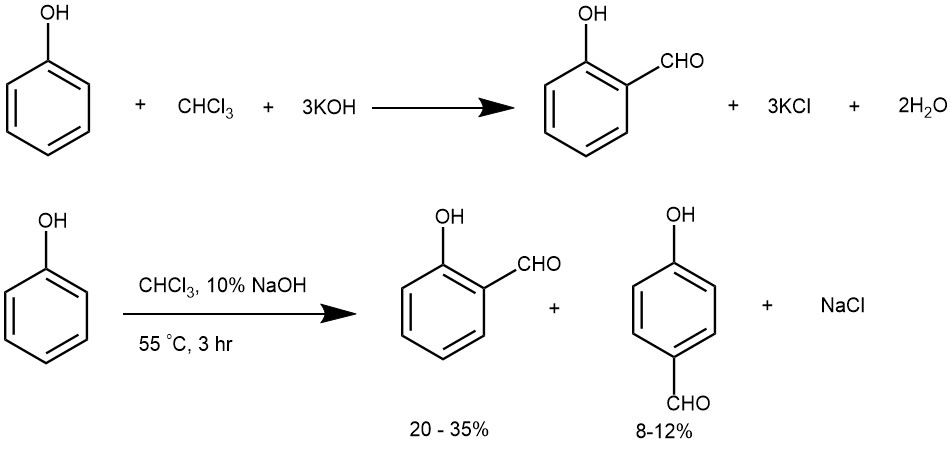
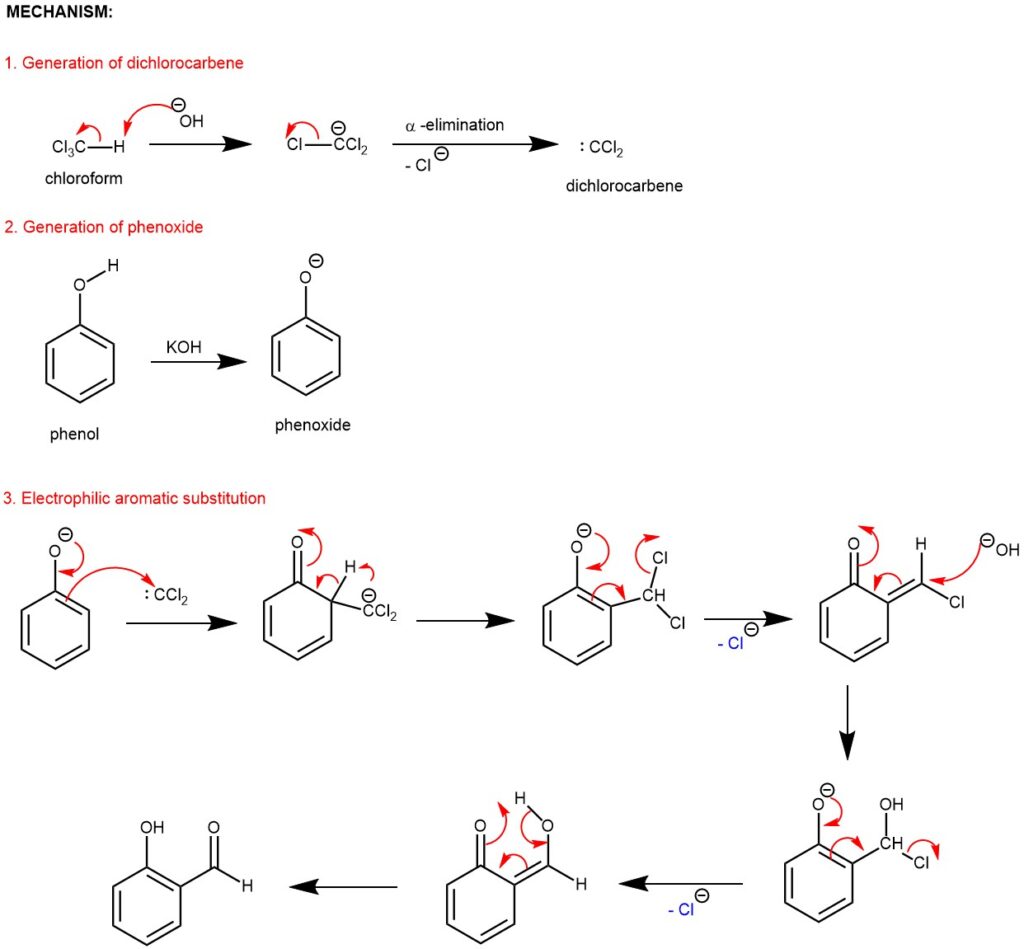
The Reimer Tiemann reaction is one of the few organic reactions that can directly formylate an aromatic ring without requiring a metal catalyst or a pre-activated substrate. It is also the only electrophilic aromatic substitution reaction that occurs under basic conditions in a protic solvent. The reaction is also unique in its selectivity for the ortho position, which is usually less favored in electrophilic aromatic substitution reactions due to steric hindrance. The selectivity can be explained by the interaction between the electron-deficient dichlorocarbene and the electron-rich phenoxide ion, which are both generated in situ from chloroform and base. The reaction mechanism most commonly involves an attack of the ortho- or para-carbon atom of the phenoxide ion on dichloroethylene ( dichlorocarbene) followed by hydrolysis and tautomerization to form the hydroxybenzaldehyde.
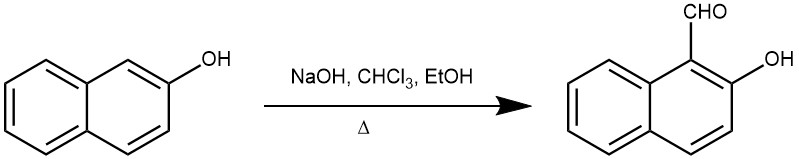
EXAMPLE 1: In a 2L three-necked round-bottomed flask fitted with a reflux condenser, a stirrer, and a dropping funnel are placed 100 g (0.69 moles) of β-naphthol and 300 g. of 95% ethanol. The stirrer is started, and a solution of 200 g. (5 moles) of sodium hydroxide in 415 g. of water is rapidly added.
The resulting solution is heated to 70–80 °C in a steam bath, and the dropwise addition of chloroform is started. After the reaction begins further heating is unnecessary. A total of 131 g. (1.1 moles) of chloroform is added at such a rate that gentle refluxing is maintained. Near the end of the addition, the sodium salt of the phenolic aldehyde separates. Stirring is continued for 1 hour after all the chloroform has been added.
The ethanol and excess chloroform are removed by distillation from a steam bath. Hydrochloric acid (sp. gr. 1.18) is added dropwise to the residue, with good stirring, until the contents of the flask are acid to Congo red paper. The dark oil that separates is mixed with a considerable amount of sodium chloride. Sufficient water to dissolve the salt is added, and the oil is separated and washed several times with hot water. By distillation of the oil under reduced pressure, there is obtained 87.5–93.7 g. of a slightly colored distillate which boils at 163–166° at 8 mm and which solidifies on cooling. Recrystallization of the solid from 75 ml. of ethanol yields 45.2–57.5 g. (38–48%) of pure 2-hydroxy-1-naphthaldehyde, melting at 79–80°.(REF: Org. Synth. 1942, 22, 63)
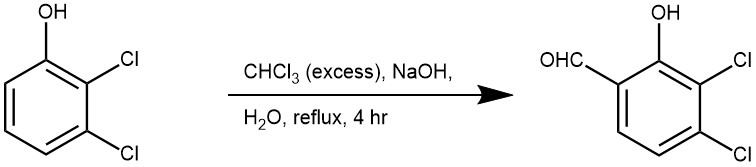
EXAMPLE 2: NaOH (24 g, 0.6 mol) was added to a solution of 2,3-dichlorophenol (16.3 g, 0.1 mol), CHCl3 (150 mL), and H2O (3.6 mL, 0.2 mol) at ambient temperature. The reaction mixture was refluxed for 4 h, diluted with H2O (250 mL) and EtOAc (100 mL), and the organic phase was separated. The aqueous phase was acidified with 1 N HCl to pH 1 and back-extracted with EtOAc. The combined extracts were washed with H2O, dried over Na2SO4, and concentrated in vacuo to give a brown oil, which was purified by column chromatography on silica gel (petroleum ether/EtOAc 10:1) to afford 8 (9.8 g, 51.3%) as a white solid:1H NMR (CDCl3 ) δ 9.88 (s, 1H, CHO), 7.45 (d, J ) 7.8 Hz, 1H), 7.18 (d, J ) 9.0 Hz, 1H); EIMS m/z 191 (M+). (REF: J. Med. Chem. 2000, 43, 4868-4876)
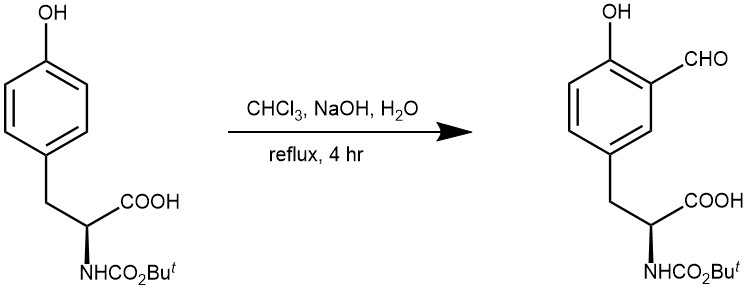
EXAMPLE 3: Powdered sodium hydroxide (1.71 g, 42.72 mmol) was added to a suspension of N-Boc L -tyrosine (7) (2 g, 7.12 mmol), water (0.256 mL, 14.13 mmol), and chloroform (30 mL). The mixture was refluxed for 4 h. Additional sodium hydroxide was added (0.42 g, 10.68 mmol) after 1 and 1.5 h. The reaction was then diluted with water and ethyl acetate, and the aqueous layer was acidified to pH 1 with 1 N HCl and back-extracted with ethyl acetate. The organic extracts were washed with brine, dried over MgSO4, and concentrated. Flash column chromatography (silica gel, 12/1 CHCl3 /MeOH 1% acetic acid eluent) afforded the desired product 8 as a brown oil (0.72 g, 33%) and recovered starting material 7 (0.62 g, 31%). The yield of 8 based on recovered starting material was 66%: 1H NMR (CDCl3, 360 MHz) δ 10.90 (1H, s, OH), 9.84 (1H, s, CHO), 7.36 (1H, s, C2-H), 7.33 (1H, d, J ) 8.3 Hz, C6-H), 6.93 (1H, d, J ) 8.3 Hz, C5-H), 5.28 (1H, bs, NH), 4.96 (1H, unresolved m, RH), 3.17 (1H, unresolved m, ‚H), 3.04 (1H, unresolved m, ‚H), 1.40 (9H, s, Boc); 13 C NMR (CDCl3, 90 MHz) δ 196.6, 175.6, 160.5, 155.3, 138.1, 134.2, 127.6, 120.4, 117.7, 80.4, 54.2, 36.8, 28.2; IR (neat) νmax 3400, 2980, 1713, 1659, 1489 cm-1. (J. Org. Chem. 1997, 62, 1553-1555)
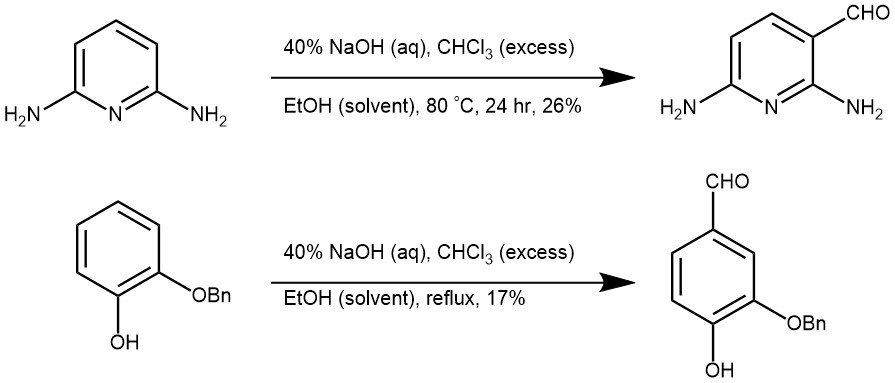
MORE EXAMPLES: (Ref: Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako)