Amines are incredibly versatile and important compounds in organic chemistry. They play crucial roles in both natural and synthetic chemistry, and their unique properties make them useful in a wide range of applications. Amines are present in many natural biologically important molecules, such as amino acids and nucleic acids, which are the building blocks of proteins and DNA, respectively. Amines are also present in many alkaloids, which are biologically active compounds found in many plants.
In addition to their roles in natural compounds, amines are also commonly used in the synthesis of synthetic compounds, including pharmaceuticals and commercial drugs. Amines are often used as bases in synthetic transformations. Amines are also important intermediates in organic synthesis, serving as building blocks for more complex molecules. They are used in the production of many common polymers, such as nylons and polyurethanes. Overall, the versatility and importance of amines make them a fascinating area of study in organic chemistry.
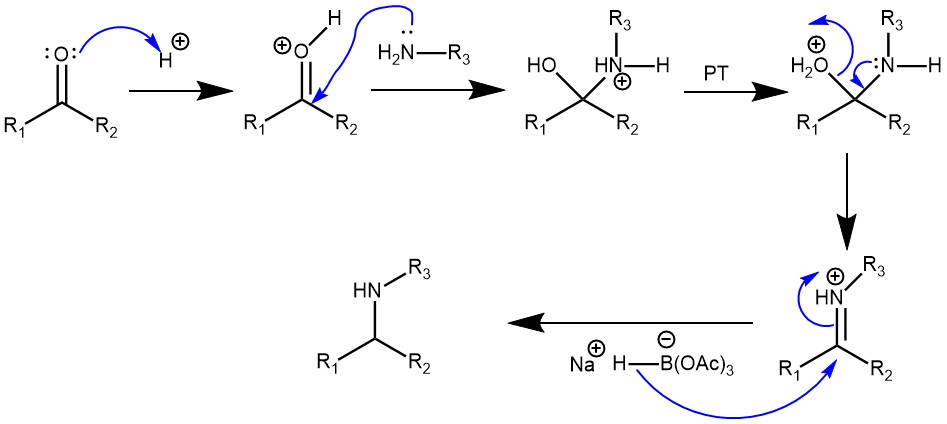
Due to these and many other importances, the synthesis of amines is of immense importance in the field of synthetic organic chemistry. One superior method of preparing amine is by alkylation of ammonia and amine by reaction of aldehyde and ketones with ammonia, primary and secondary amines in the presence of a reducing agent to form the primary, secondary, and tertiary amines respectively. This method is referred to as the Reductive Amination of carbonyl compounds. The reductive amination of aldehydes and ketones is a cornerstone reaction and is one of the most useful and important tools in the synthesis of different kinds of amines. Generally, the reaction proceeds via the initial formation of an intermediate carbinolamine, which dehydrates to form an imine (Schiff base) or iminium ion. Reduction of which produces the amine product.
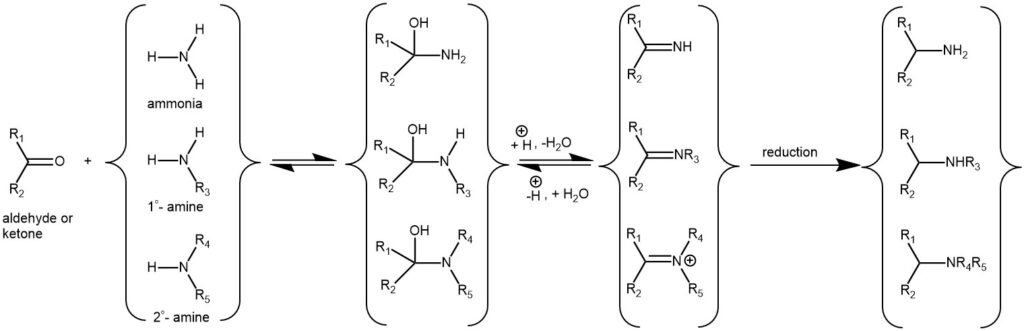
The reductive amination reaction may be classified as Direct when the carbonyl compound and the amine are mixed along with the suitable reducing agent without the prior formation of the imine or the iminium ion. Selection of the reducing agent is critical as the reducing agent should selectively reduce the imine or the iminium ion but not the carbonyl compounds under the same reaction conditions. On the other hand, the Indirect method involves the preformation of the imine or iminium ion followed by the addition of the reducing agent. The choice of the reducing agent is not very critical.
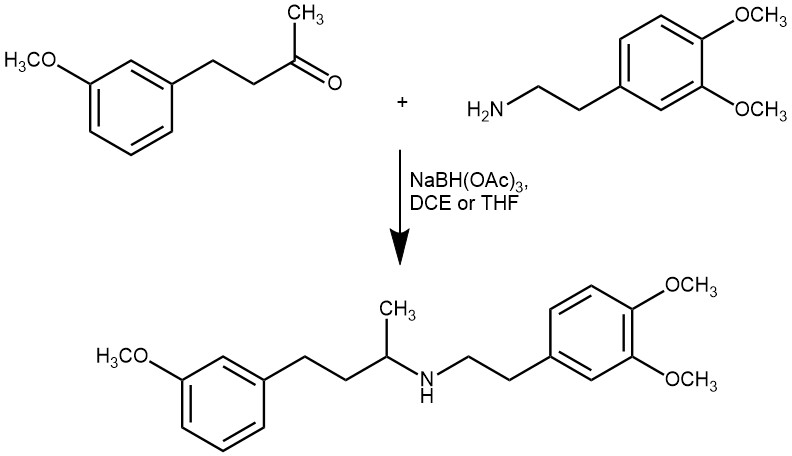
Sodium triacetoxyborohydride (STAB) is one such selective reducing agent for direct reductive amination. It is a mild reagent that possesses remarkable selectivity as a reducing agent. The steric and electron-withdrawing effects of the three acetoxy groups stabilize the boron-hydrogen bond and are responsible for its mild reducing properties. It is commercially available as a hygroscopic white powder with a melting point of 116-120°C. It is also easily prepared by the reaction of NaBH4 with excess acetic acid in benzene or toluene.
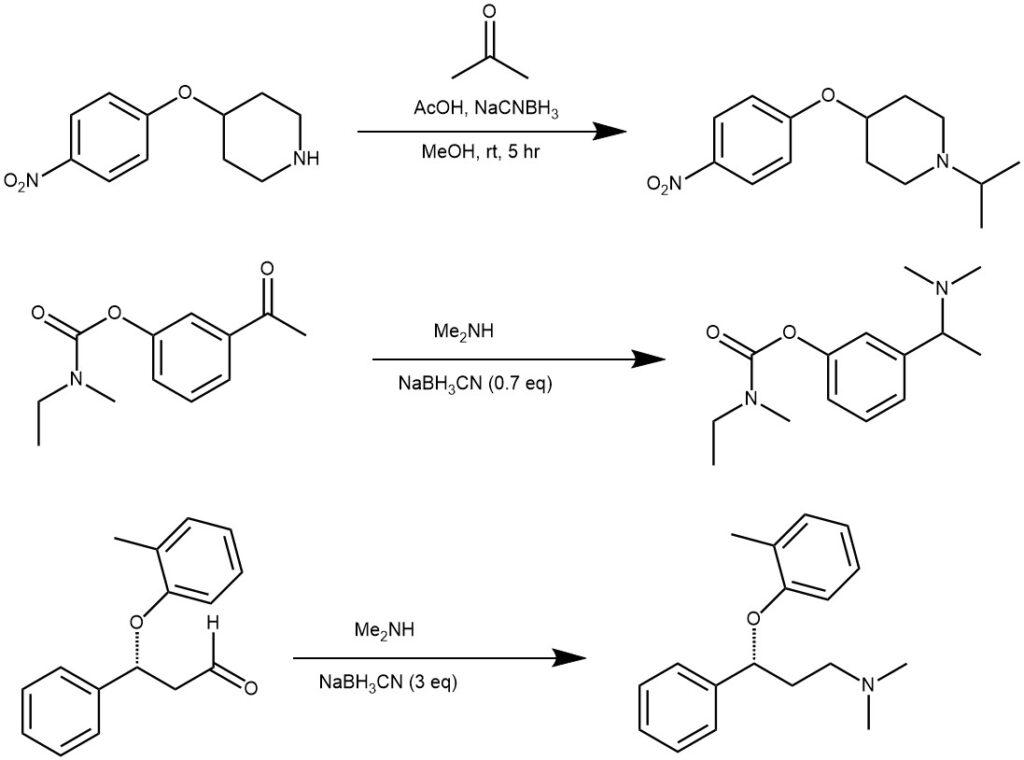
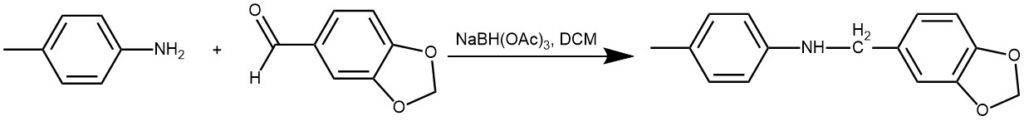
EXAMPLE 1: A solution of the carbonyl compound (1.3 mmol) and the amine (1.9 mmol) is prepared by dissolving in dichloromethane (35 mL). STAB (2 equivalent) is then added to the solution and stirred. After the completion of the reaction, it is quenched with aqueous NaHCO3 and extracted with DCM. It is further purified by crystallization in hot ethanol.
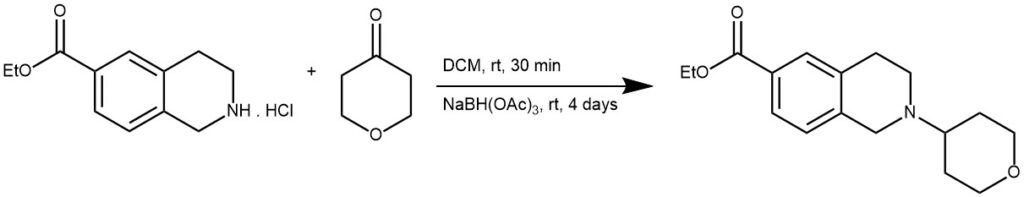
EXAMPLE 2: To a solution of the amine hydrochloride (14.3 mmol) in DCM (150 mL) was added the ketone (20 mmol). The mixture was stirred at room temperature for 30 minutes. STAB was then added (56 mmol) and stirred at room temperature for 4 days. It was then quenched with sat. aq. NaHCO3 and extracted with DCM. It was further purified by column chromatography.
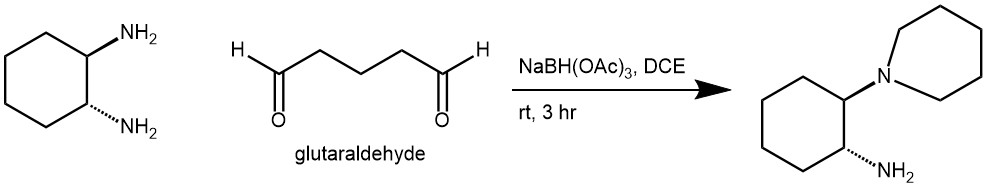
EXAMPLE 3: Glutaraldehyde (5.1 mmol) was added dropwise into a mixture of amine (4.90 mmol) and STAB (19.6 mmol) in DCE (30 mL) at room temperature. The mixture was stirred at room temperature for 3 hours and then quenched with aq. NaOH solution (6M, 15 mL). The organic layer was separated and the aqueous layer was extracted with DCM. The combined organic layer was evaporated. The residue was dissolved in 20 mL of DCM, washed with brine, dried over sodium sulfate, filtered, and concentrated to give the product (89% yield).
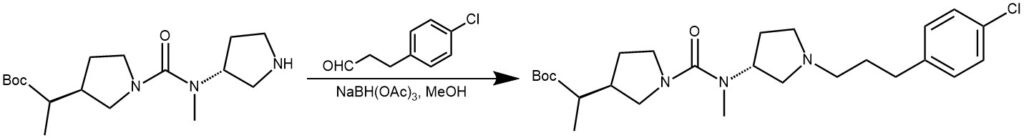
EXAMPLE 4: To the bis-pyrrolidine (19 mmol) in MeOH (80 mL) stirred in an ice bath was added STAB (38 mmol) and acetic acid in a catalytic amount. To this reaction mixture was added 4-chlorohydrocinnamaldehyde (38 mmol) with stirring for 0.5 hours. Quenching of the reaction employing water preceded removal of the solvent under vacuum. The residue was diluted with DCM: i-PrOH (3:1), and washed three times with sat. NaHCO3, twice with brine, and then dried over MgSO4 before filtration and concentration to yield an oil. Crystallization from ether/hexane was followed.
The other reducing agent used most often for reductive amination is sodium cyanoborohydride (NaBH3CN). The successful use of sodium cyanoborohydride is due to its stability in relatively strong acid solutions (~ pH 3), its solubility in hydroxylic solvents like methanol, and its different selectivities at different pH values. At pH 3-4, it reduces aldehydes and ketones effectively, but this reduction becomes very slow at higher pH values. At pH 6-8, the more basic imines are protonated preferentially and reduced faster than aldehydes or ketones. This selectivity allows for a convenient direct reductive amination procedure.