Alcohols are a ubiquitous and versatile class of compounds in organic chemistry. They are present in natural products, pharmaceuticals, etc., and are ubiquitous synthetic handles for a multitude of organic transformations.
The undergraduate and graduate courses are full of reactions that can transform alcohols into different other functional groups. Oxidation of alcohols to carbonyl compounds is one such important transformation. However, barely any reactions related to the reduction of alcohol are encountered in undergraduate courses.
One of the earliest methods for alcohol deoxygenation is the Barton-McCombie radical deoxygenation. However, owing to the higher bond strength of the C-OH bond, cleavage of this bond is a difficult maneuver. Barton-McCombie reaction requires pre-derivatization of the alcohol to O-alkylthiocarbonyl. In a typical procedure the alcohol is first converted into a thiocarbonyl derivative, which is then exposed to the tri-n-butyltin hydride in refluxing toluene.
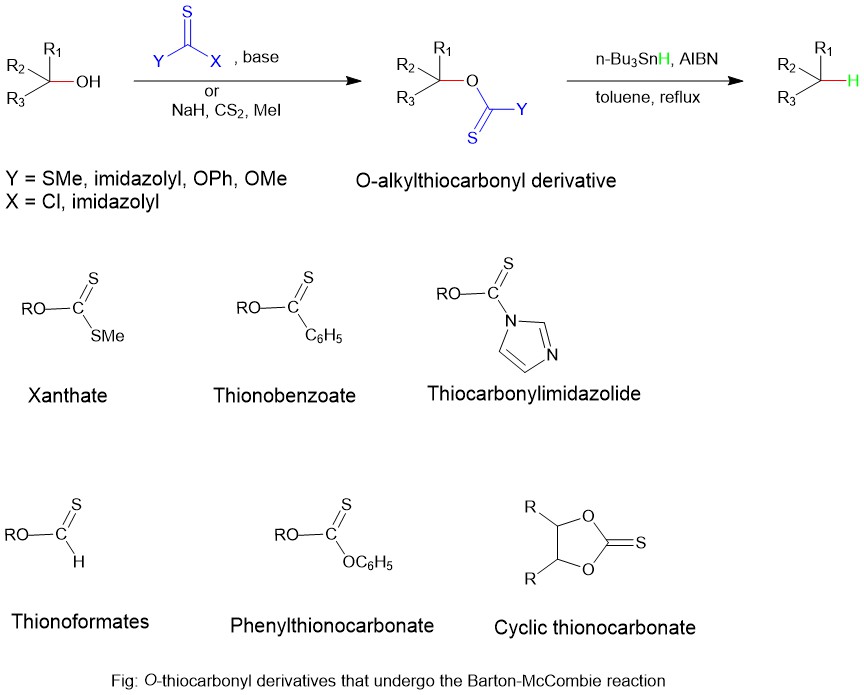
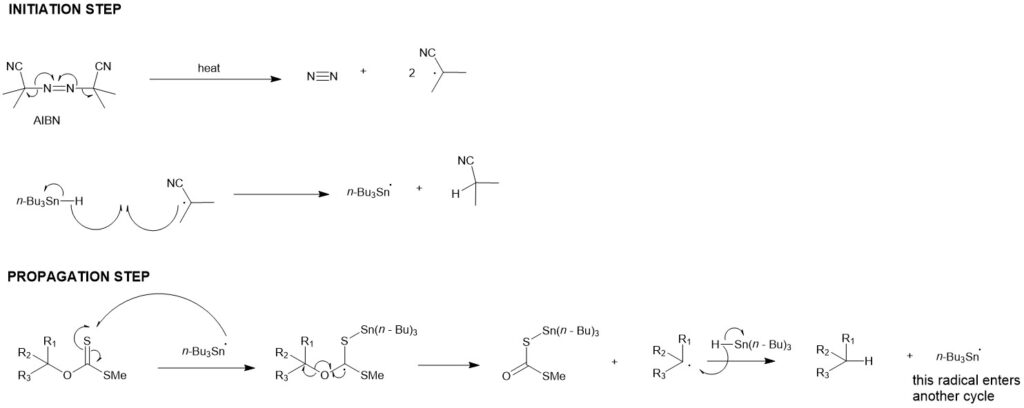
MECHANISM:
The Barton-McCombie reaction is a pioneering radical deoxygenation reaction. In 1975, they reported a two-step sequence for hydroxyl-group (-OH) replacement by a hydrogen atom. The first step is the conversion of the hydroxyl group into an O-thiocarbonyl group, and the second step is the free-radical chain reaction that replaces the O-thiocarbonyl group with a hydrogen atom. The driving force is the formation of a very stable S-Sn bond. In addition, tin hydrides are good hydrogen atom donors
Eg.