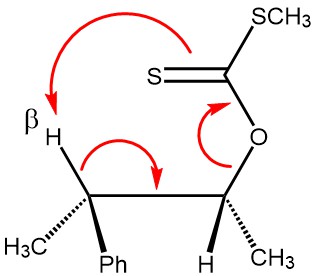
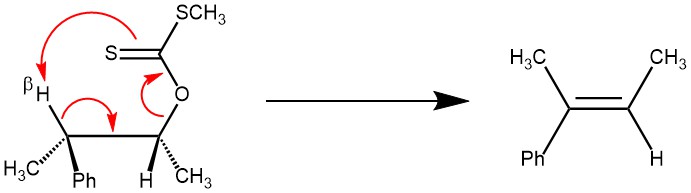
The pyrolytic elimination or Ei (elimination internal/intramolecular) mechanism is a special kind of elimination reaction where two vicinal groups on an alkane framework leave simultaneously through a cyclic transition state to form an alkene.
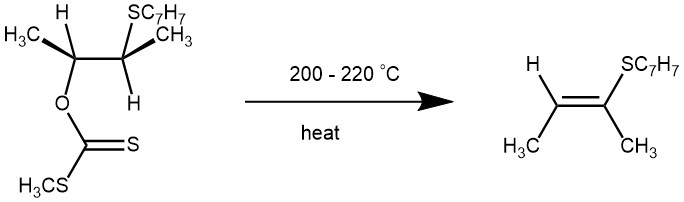
The formation of olefins by pyrolysis of the corresponding xanthates (containing at least one β-hydrogen) via cis-elimination is known as the Chugaev elimination reaction. It is named for its discoverer, the Russian chemist Lev Aleksandrovich Chugaev (1873-1922), who first reported the reaction sequence in 1899. The overall reaction involves the decomposition of a xanthate into an olefin with no rearrangement.
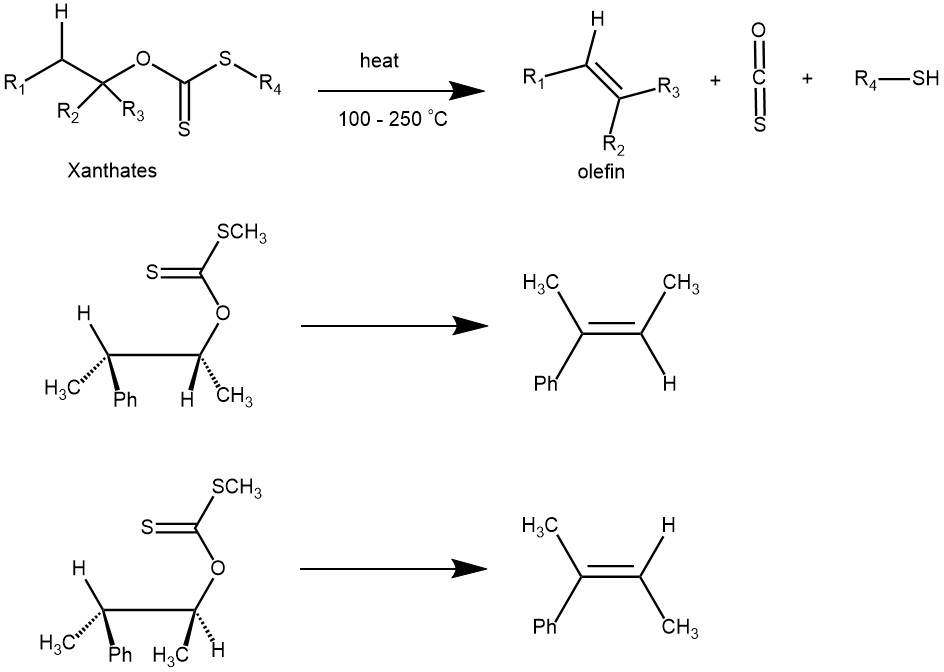
PREPARATION OF XANTHATES: Typically xanthates are prepared from the alcohols (1°, 2°, or 3°) by first deprotonating the alcohol with a base (eg. NaH, NaOH, KOH) and reacting the resulting alkoxide with carbon disulfide. It is then trapped with an alkyl iodide

MECHANISM: The Chugaev reaction is an intramolecular syn-elimination (Ei), and proceeds through a six-membered transition state involving a cis-β-hydrogen atom of the alcohol moiety and the thione sulfur atom of the xanthate. The β-hydrogen and the xanthate group must be coplanar in the cyclic transition state.
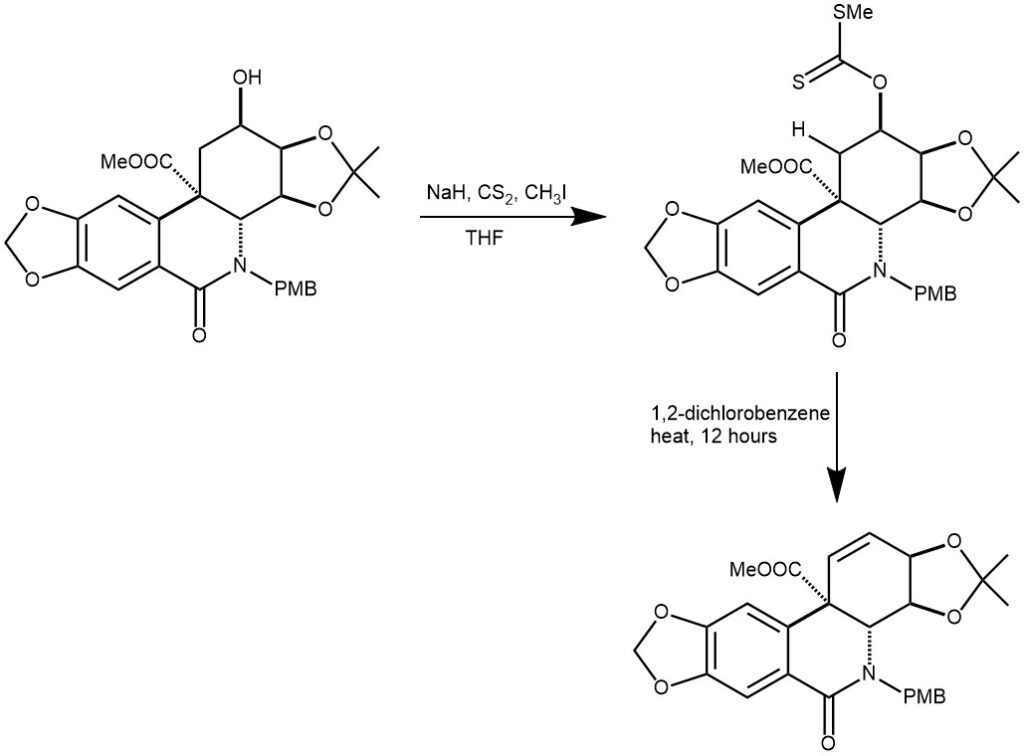
EXAMPLE 1: To a solution of 0.40 g (0.7 mmol) of alcohol in 7 mL of THF was added 0.09 g (2.2 mmol)of NaH (60% in mineral oil) at 0 °C. After stirring for 10 min, the mixture was warmed to room temperature and was stirred for an additional 30 min. The solution was cooled to 0 °C, and 0.26 mL (4.4 mmol) of CS2 was added and the mixture was stirred for 1 h at this temperature and then 0.5 mL (8.8 mmol) of Mel was added. After stirring for 10 min, the mixture was warmed to room temperature and was stirred for an additional 20 min. The solution was quenched with a saturated NH4Cl solution and then extracted with EtOAc. The combined organic extracts were washed with H2O, brine, and dried over MgSO4. After concentration under reduced pressure, the residue was dissolved in 20 mL of 1,2-dichlorobenzene and was heated at reflux for 12 h. After cooling to room temperature, the mixture was concentrated under reduced pressure and subjected to flash silica gel chromatography to give 0.34 g (94%)of alkene 43 as a pale yellow oil.[REF: J. Org. Chem., 2007, 72, 2570-2582]
EXAMPLE 2: dl-threo-3-p-Tolylthio-2-butanol (9.8 g., 0.05 mole) was added to a stirred suspension of 1.95 g. (0.05 g. atom) of metallic potassium in 100 ml. of dry benzene. The mixture was stirred for 2 hr. at room temperature and then for 2 hr. at 50-55°. All the potassium appeared to have reacted at the end of this period. Carbon disulfide (15 ml.) was added, the solution was refluxed for 8 hr. and 20 ml. of methyl iodide was added. The solution was stirred overnight, filtered and washed thoroughly with water. After drying over anhydrous sodium sulfate, the solution was filtered and the solvent removed, first on a steam-bath, then under vacuum. The residual oil weighed 13.5 g. (94%). The crude xanthate (7.0 g., 0.022 mole), was placed in a 25-ml. vacuum distillation flask fitted with a3- inch Vigreux head. The flask was heated at 200° for 30 min., vacuum was applied slowly and the product was distilled at 0.1 mm. keeping the pot temperature at 200-220°.In this way 3 g. (77%) of an oil, b.p. 135-147° at 0.1 mm., was collected. [REF: J. Am. Chem. Soc., 1958, 80, 23, 6383-6386]
Reference:
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako