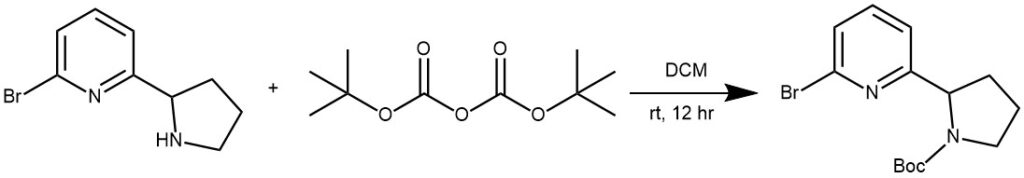
Amines are reactive functional groups that can participate in a variety of chemical reactions. While this reactivity can be advantageous in some reactions, it can also be problematic in others. For example, amines can react with other reagents or functional groups in the reaction mixture, leading to unwanted side reactions or products. To prevent these unwanted reactions and products, it is often necessary to protect the amine functional group during chemical synthesis. This can be achieved by modifying the amine in a way that temporarily blocks its reactivity while allowing the desired reaction to proceed. This temporary modification is often referred to as a protecting group or a “protection.”
The presence of the amine functionality in a wide range of biologically active compounds makes the protection of amines an important and frequently needed exercise in synthetic organic/medicinal chemistry. Protection of the amino group is one of the most fundamental and useful transformations during the synthesis of peptides, amino acids, and other natural products. Amines can be protected using various chemical methods to prevent unwanted reactions from occurring during a synthesis. Here are some of the most common methods of protecting amines:
Acylation: Amines can be protected by acylating them with an acid chloride or an anhydride to form an amide.
Alkylation: Amines can be protected by alkylating them with an alkyl halide to form a tertiary amine.
Tosylation: Amines can be protected by reacting them with p-toluenesulfonyl chloride (tosyl chloride) to form a tosylate.
Benzylation: Amines can be protected by reacting them with benzyl chloride to form a benzylamine.
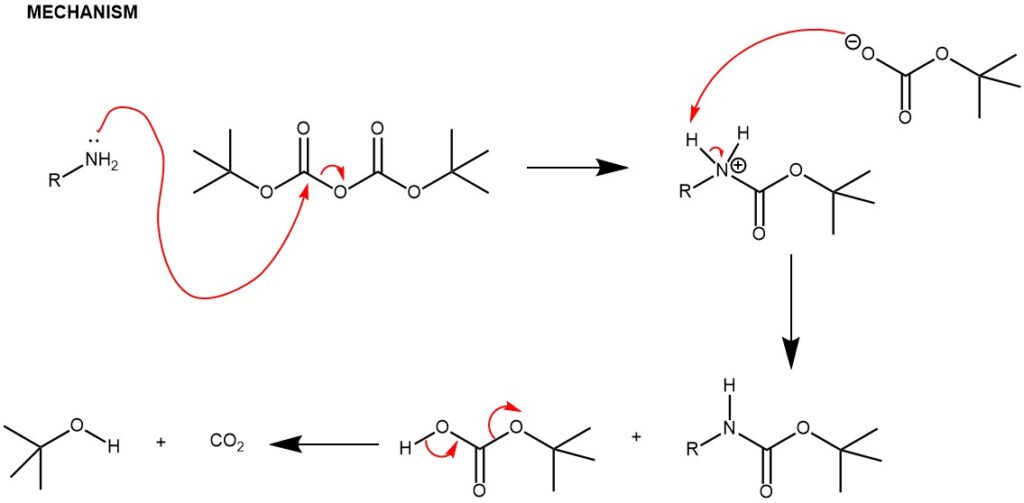
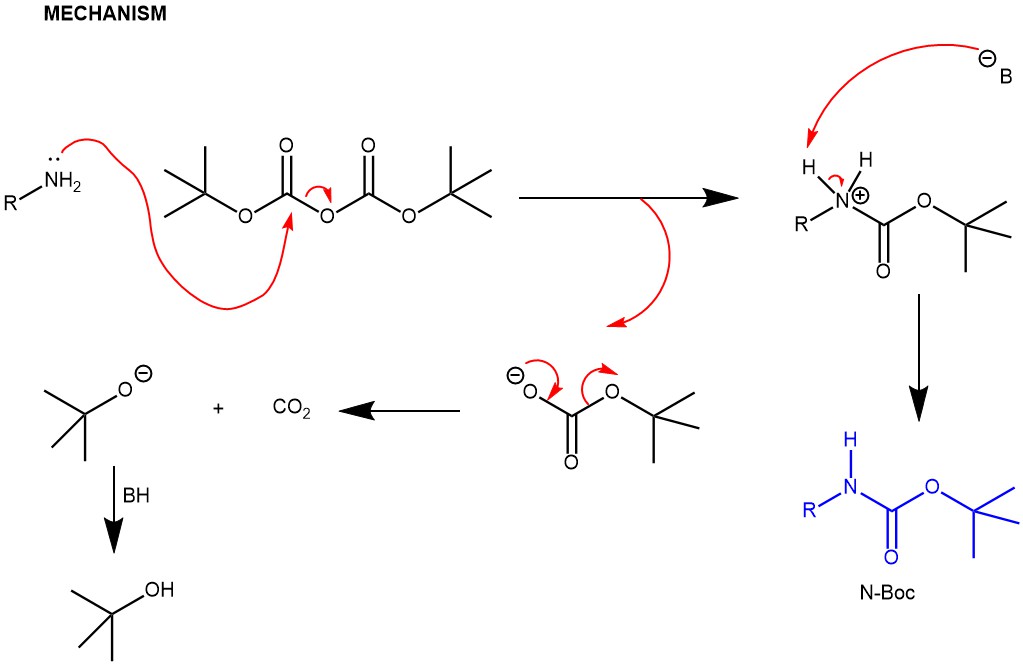
Carbamation: Amines can be protected by reacting them with di-tert-butyl dicarbonate (Boc)2O to form a carbamate. The resulting carbamate is stable and will not react with other reagents during the synthesis. Due to the stability toward catalytic hydrogenation and resistance toward basic conditions and many other nucleophilic reagents, the tert-butoxycarbonyl (Boc) group is employed extensively to mask the amino function, finding widespread use in both organic and peptide synthesis. Amines are converted to N-tert-Boc derivatives by reaction with di-tert-butyl dicarbonate [(Boc)2O], usually in the presence of DMAP and with or without bases
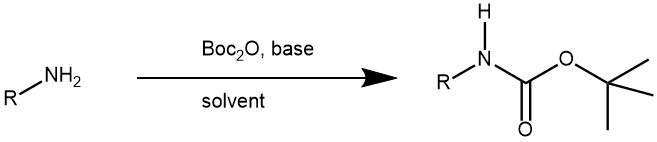
1. Amine protection with di-tert-butyl dicarbonate:
EXAMPLE 1: To a solution of 2-bromo-6-(pyrrolidin-2-yl)pyridine (250 mg, 1.1 mmol, 1.0 eq) in 10 mL of DCM was added (Boc)20 (1.2 g, 5.5 mmol, 5.0 eq). The reaction mixture was stirred at rt for 12 h and then concentrated in a vacuo. The residue was purified by silica gel chromatography with PE/EA (10/1) as eluent to give tert-butyl 2-(6-bromopyridin-2- yl)pyrrolidine-l-carboxylate. 330 mg, as white solid in 90% yield. (Ref: World Intellectual Property Organization, WO2016011390 A1 2016-01-21)
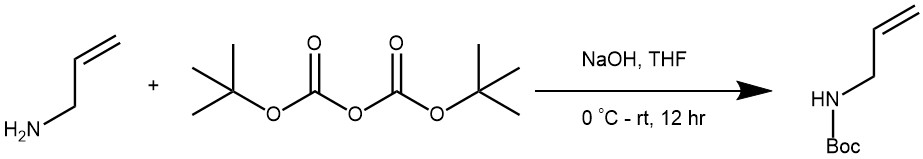
2. Amine protection with di-tert-butyl dicarbonate in presence of a base
EXAMPLE 1: To a solution of 3-Aminopropylene (2.62 ml, 35.0 mmol, 1.0 equiv) in THF (20 mL) at 0° C was added NaOH aqueous solution (16.00 ml, 35.0 mmol, 2 mol/L) for 30 min. Then a solution of Boc anhydride (8.04 ml, 35.0 mmol, 1.0 equiv) in 20 mL THF was added dropwise by constant pressure funnel under vigorous stirring. The mixture was stirred at room temperature overnight and then washed with water (50 mL x 3). The organic layer was dried over Na2SO4 and concentrated to afford the crude (5.00 g, 31.8 mmol, 91% yield), which was used without further purification. (Org. Lett. 2017, 19, 1, 86–89)
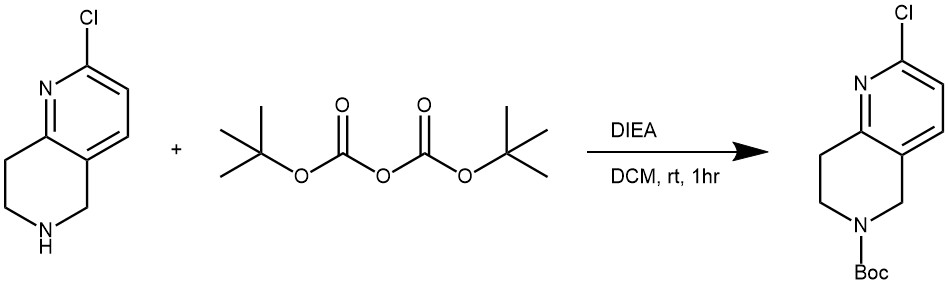
EXAMPLE 2: To a slurry of 2-chloro-5,6,7,8-tetrahydro-l,6-naphthyridine hydrochloride (106.1 g, 517 mmol and N,N-diisopropylethylamine (80 g, 108 mL, 621 mmol, 1.2 eq) in DCM (1 L) was added a solution of di-tert-butyl dicarbonate (119 g, 543 mmol, 1.05 eq) in DCM (100 mL) via an addition funnel within 1 hr. The reaction mixture became a clean solution and the solution thus obtained was stirred at room temperature for an additional hour and monitored using LCMS. Upon completion, the reaction mixture was concentrated. The residue was dissolved in EtOAc (1 L) and washed with water (3 x 300 mL), washed with brine (300 mL), and dried over MgSO4. The solvent was evaporated under vacuum to give the title compound as an off-white solid (139 g, yield: 100%). (World Intellectual Property Organization, WO2012129344 A1 2012-09-27)
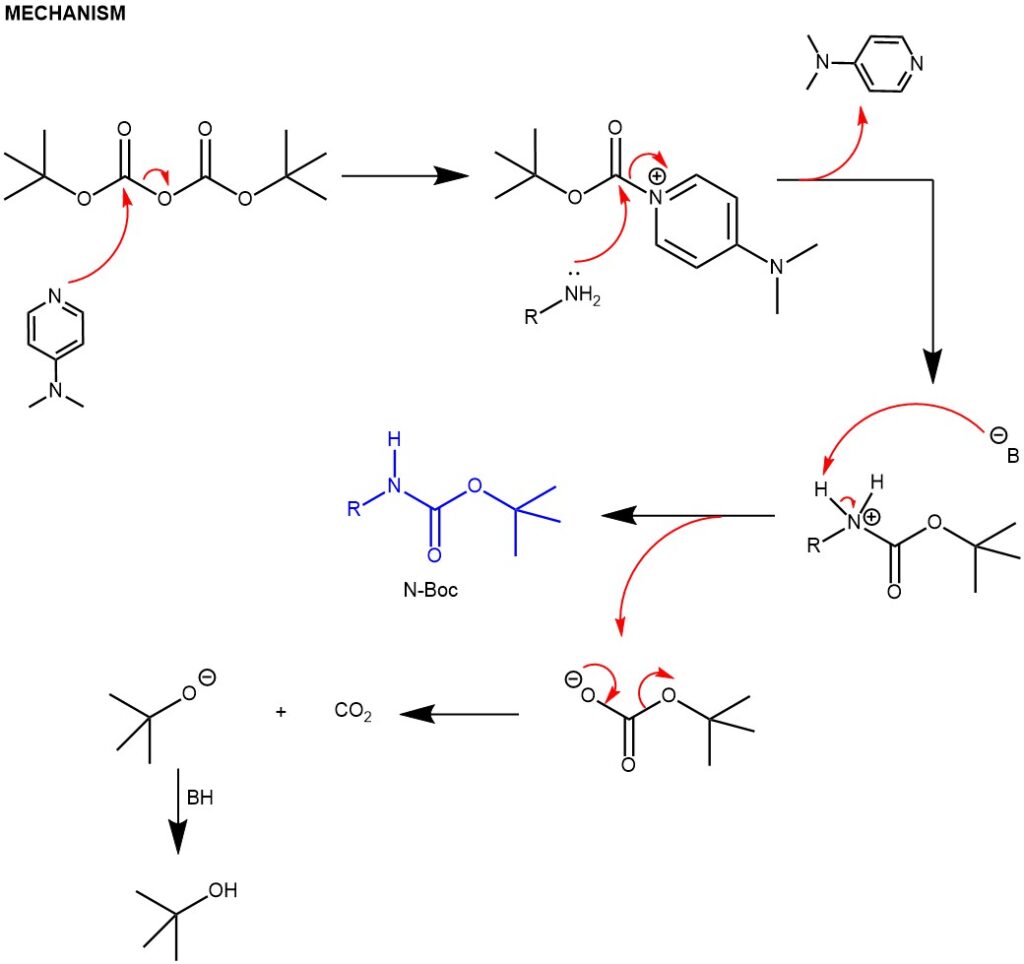
3. Amine protection with di-tert-butyl dicarbonate in presence of 4-(N,N-dimethylamino)pyridine (DMAP).
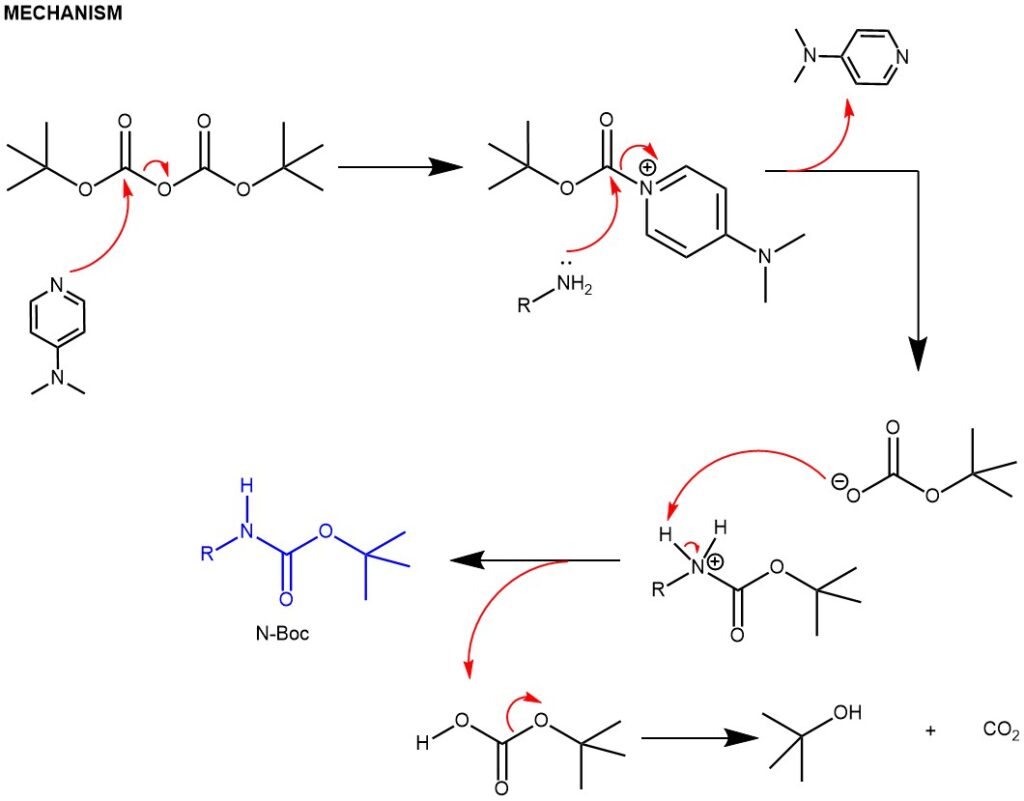
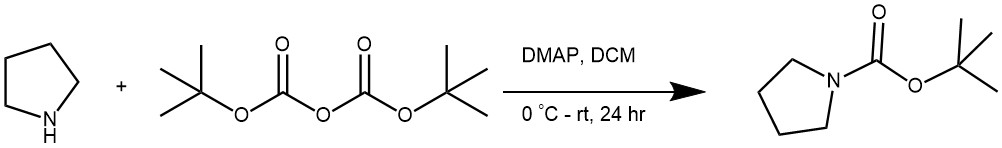
EXAMPLE 1: First, pyrrolidine (10 mmol, 0.906 mL) and 4-DMAP (1 mmol, 12.2 mg) are dissolved in dichloromethane (30 mL) in a flask under an argon atmosphere. The mixture is cooled to 0 °C and a solution of (Boc)2O (11 mmol, 2.40 g) in dichloromethane (5mL) is added dropwise to the mixture at this temperature. After 10 minutes, the mixture is allowed to reach room temperature and stirred for 24 hours. The resulting solution is then washed with water and brine, and the organic phase is dried over sodium sulfate . The solvent is removed under reduced pressure and the resulting crude product is purified by column chromatography with petroleum ether/ethyl acetate (90/10) as the eluent to obtain tert-butyl pyrrolidine-1-carboxylate. It is important to follow proper safety measures and conduct this procedure only in a properly equipped laboratory. (A European Journal (2019), 25(70), 16120-16127)
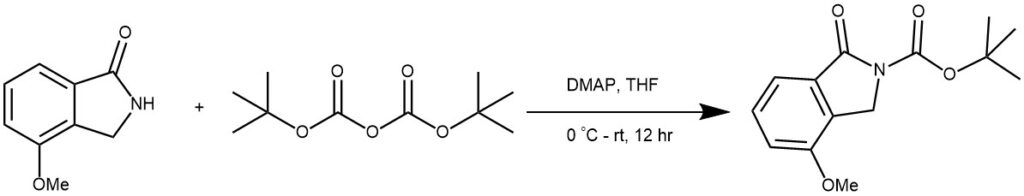
EXAMPLE 2: First, DMAP (0.2 mmol) and (Boc)2O (5.0 mmol) are added to a solution of the reactant (2.0 mmol) in dry THF (10 mL). The mixture is stirred at room temperature for 12 hours. Next, the mixture is quenched with NH4Cl and extracted with EtOAc (30 mL × 3). The combined organic layers are washed with brine, dried over MgSO4, filtered, and concentrated. Finally, the residue is purified by flash chromatography on silica gel using PE/EA (5:1) as the eluent.
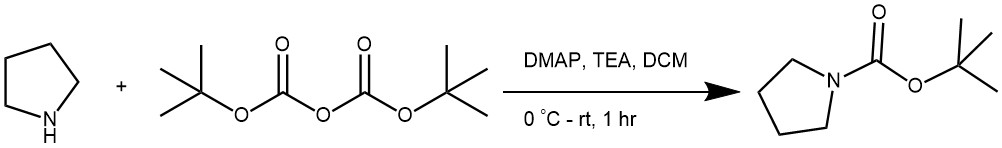
Amine protection with di-tert-butyl dicarbonate in presence of 4-(N,N-dimethylamino)pyridine (DMAP) and a base
EXAMPLE 1: To a stirred mixture of pyrrolidine (10.0 g, 0.14 mol) in DCM (150 mL) at 0° C was added TEA (15.6 g, 0.15 tool) followed by (Boc)2O (30.6 g, 0.14 mol), and the mixture was stirred at RT for 1 hour, TLC showed that pyrrolidine had disappeared. The mixture was washed with a 1 M aqueous HCl solution (100 mL), brine, and dried over sodium sulfate, filtered, and concentrated in vacuo to give the product. Colorless oil, yield 24.0 g, 100%. (World Intellectual Property Organization WO2013102242).
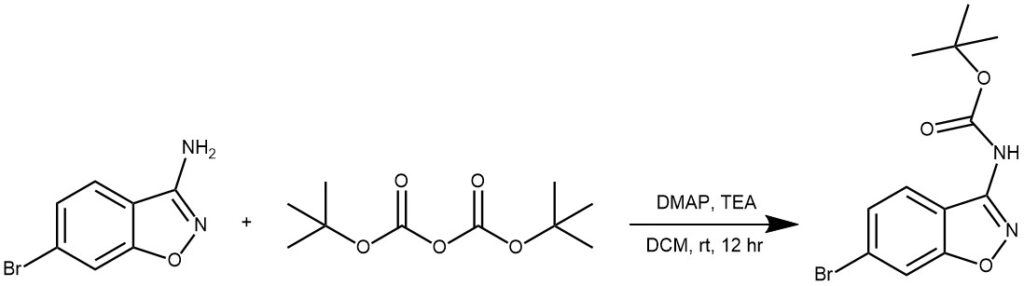
EXAMPLE 2: A mixture of the SM (900 mg, 4.25 mmol), (Boc)2O (1.2 g, 5.1 mmol), TEA (686 mg, 6.8 mmol), and DMAP (72 mg, 0.6 mmol) in DCM (10 mL) was stirred at RT overnight. H2O was added and the layers were separated. The aq layer was further extracted with DCM. The combined organics were washed with brine, dried (Na2SO4), and concentrated. The crude material was purified by silica gel column chromatography to provide the product as a solid. [900 mg, 68%]