The Pinacol-pinacolone rearrangement is a notable organic reaction that involves the rearrangement of a pinacol (1,2-diol) to form a pinacolone (ketone/aldehyde) compound. This reaction was first discovered by Wilhelm Rudolph Fittig in 1860. This rearrangement involves a 1,2-shift of substituents with concomitant formation of a C-H or C-C bond α to a carbonyl group. The name of this rearrangement comes from the rearrangement of pinacol to pinacolone.
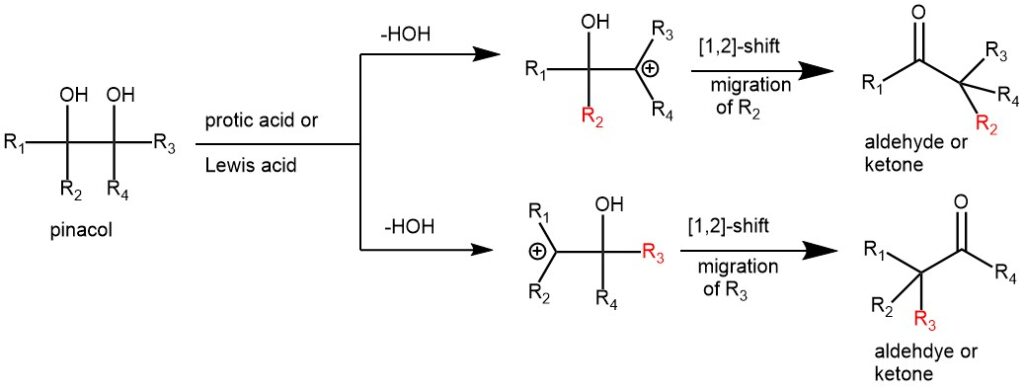
Virtually any cyclic or acyclic vicinal diols (1,2-diol or glycols) can undergo rearrangement, and, depending on the substituents pattern, aldehydes and/or ketones are formed. When the glycol is unsymmetrical the product is resulted via the most stable carbocation intermediate. The substituent that is able to stabilize a positive charge better (better electron donor) tends to migrate preferentially. The relative migration aptitude is aryl ~ H ~ vinyl (alkenyl) > t-butyl >> cyclopropyl > 2° alkyls> 1° alkyl. Besides sulfuric acid and other protic acids, Lewis acids like BF3.OEt2 and TMSOTf are also used.
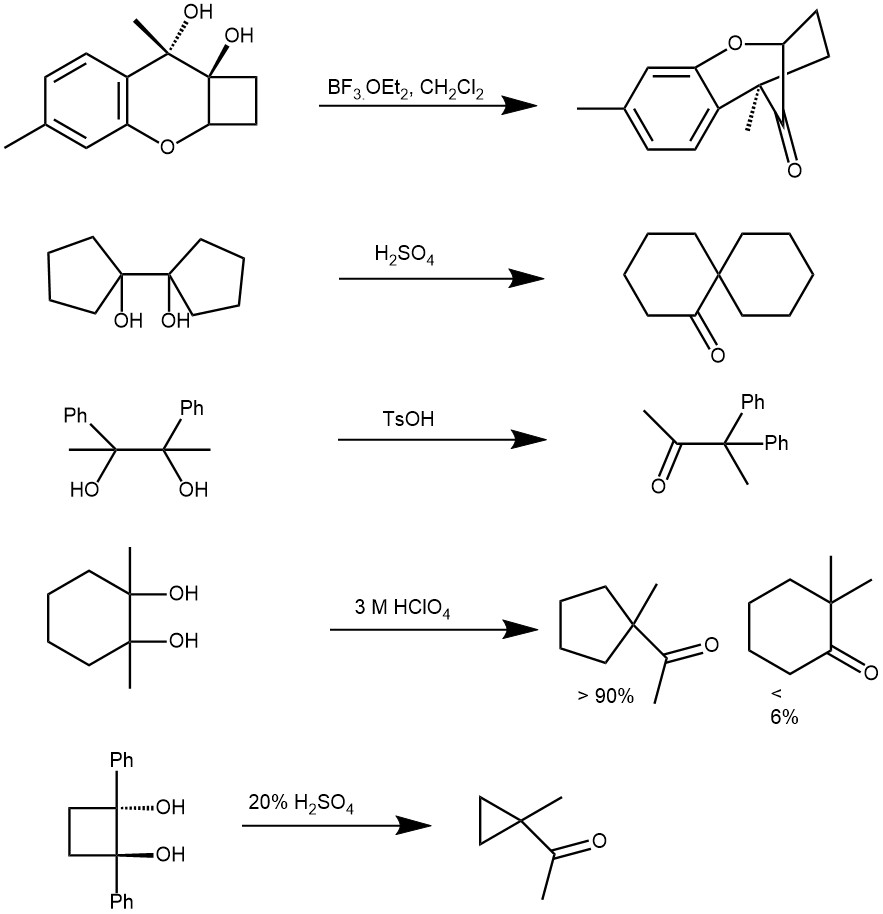
MECHANISM: The first step is the protonation of one of the hydroxyl groups followed by the departure of a water molecule forming a carbocation intermediate. This intermediate then undergoes a [1,2]-shift to form a more stable carbocation intermediate. This then loses a proton to afford the final pinacolone product.

EXAMPLE 1: BF3.OEt2 (0.10 mL; 0.81 mmol; 4 equiv) was added dropwise to a stirred solution of (R,R)-diol (0.11 g; 0.18 mmol) and 2,2-DMP (0.19 mL; 1.5 mmol; 8 equiv) in acetone (2 mL) under argon at room temperature. After 1 h, saturated NaHCO3 (aq) was added to the mixture before extraction with DCM (4 × 5 mL) and drying of the combined organics. Evaporation of the solvent in vacuo afforded a light brown oil, which was subject to flash column chromatography (15:1 hexanes-EtOAc) to generate a clear oil that crystallized from CH3OH as a colorless solid (0.040 g; 39%): mp 103-104 °C; Rf 0.15 (10:1, hexanes-EtOAc)[REF: J. Org. Chem., Vol. 65, No. 22, 2000]

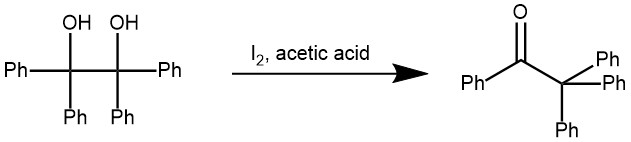
EXAMPLE 2: In a 1L round-bottomed flask provided with a reflux condenser is placed a solution of 1 g. of iodine in 500 cc. of glacial acetic acid. One hundred grams (0.27 mole) of benzopinacol is added, and the flask is heated over a wire gauze, with shaking, until the solution boils gently. It is then refluxed for five minutes during which the solid benzopinacol disappears completely and a clear red solution isobtained. The solution is transferred at once to a 1L beaker, and, upon cooling, the benzopinacolone separates in fine threads. The product is filtered with suction, washed with two or three 60-cc. portions of cold glacial acetic acid until colorless, and dried. The filtrate is reserved for subsequent preparations. The yield of practically pure benzopinacolone melting at 178–179° is 90–91 g (95–96 % of the theoretical amount). If a purer product is desired the material may be dissolved in 450 cc. of hot benzene, filtered, and treated with 250 cc. of hot ligroin (b.p. 90–100°). After cooling in ice the benzopinacolone is filtered and dried. The purified product weighs 82–83 g. and melts at 179–180°.
To the acetic acid filtrate is added another 100-g. portion of benzopinacol and the reaction is carried out in the same way. The yield of benzopinacolone in the second and subsequent runs is 94–94.5 g. (98–99 % of the theoretical amount). This procedure can be repeated in the same filtrate until 500 g. of the pinacol has been rearranged.[REF: Organic Syntheses, Coll. Vol. 2, p.73 (1943); Vol. 14, p.12 (1934)]
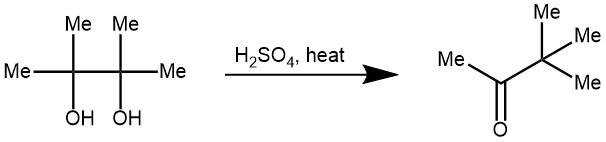
EXAMPLE 3: In a 2L round-bottomed flask, fitted with a stopper carrying a dropping funnel and a connection to a condenser set for distillation, are placed 750 g. of 6 N sulfuric acid and 250 g. of pinacol Hydrate. The mixture is then distilled until the upper layer of distillate ceases to increase in Volume. This requires about fifteen to twenty minutes. The pinacolone layer in the distillate is separated from the water and the water is returned to the reaction flask. First, 60 cc. of concentrated sulfuric acid is added to the water, and then a second 250-g portion of pinacol hydrate. The distillation is repeated. This process is repeated twice more until 1 kg. (4.42 moles) of pinacol hydrate has been used. The combined pinacolone fraction is dried over calcium chloride, filtered, and fractionally distilled. There is first a small low-boiling portion; then the pinacolone comes over at 103–107°; and finally, there is a higher-boiling portion which yields more pinacolone on redistillation. The yield from a run, as described, is 287–318 g. (65–72 percent of the theoretical amount). This product occasionally turns slightly yellow on standing, but redistillation removes the color with almost no loss of product.[REF: Organic Syntheses, Coll. Vol. 1, p.462 (1941); Vol. 5, p.91 (1925)]
REFERENCES;
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako
- Pinacol rearrangement. (2023, June 28). In Wikipedia. https://en.wikipedia.org/wiki/Pinacol_rearrangement



