The reaction is named after Russian chemist Nikolay Prilezhaev, who first reported the reaction in 1909. The Prilezhaev reaction, also known as the Prilezhaev epoxidation, is a chemical reaction that involves the conversion of an alkene to an epoxide using an organic peroxy acid (also called peracid), such as meta-chloroperbenzoic acid (mCPBA) or peracetic acid. Epoxides, also known as oxiranes, are three-membered cyclic ethers that are widely used in organic synthesis. The Prilezhaev reaction has important applications in organic synthesis, particularly in the production of various types of epoxides that are used as intermediates in the synthesis of other organic compounds. It is also used as a key step in the production of various pharmaceuticals, such as the anti-cancer drug paclitaxel.
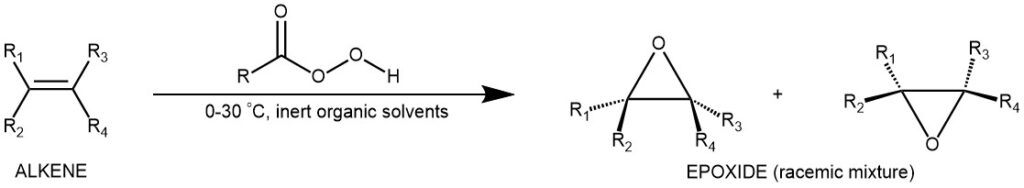
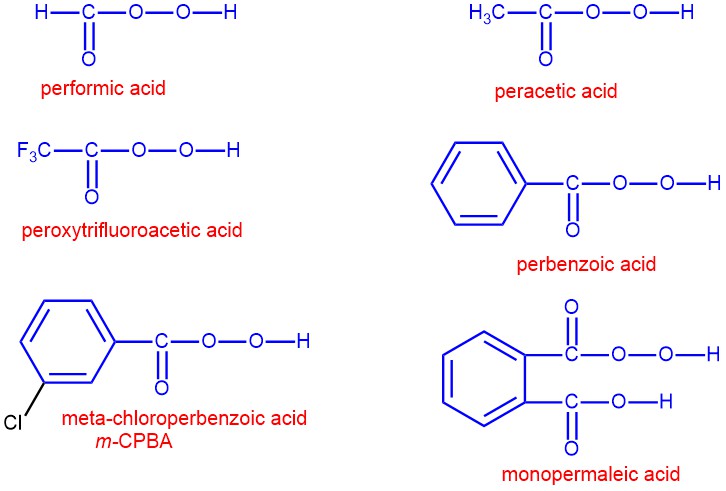
Peroxy acids are carboxylic acids that have an extra oxygen atom in the -COOH group, forming a -COOOH group. They are strong oxidizing agents that can transfer an oxygen atom to other molecules. Some examples of peroxy acids are peracetic acid (CH3COOOH), perbenzoic acid (C6H5COOOH), and m-chloroperoxybenzoic acid (m–CPBA), peroxytrifluoroacetic acid (CF3COOOH).
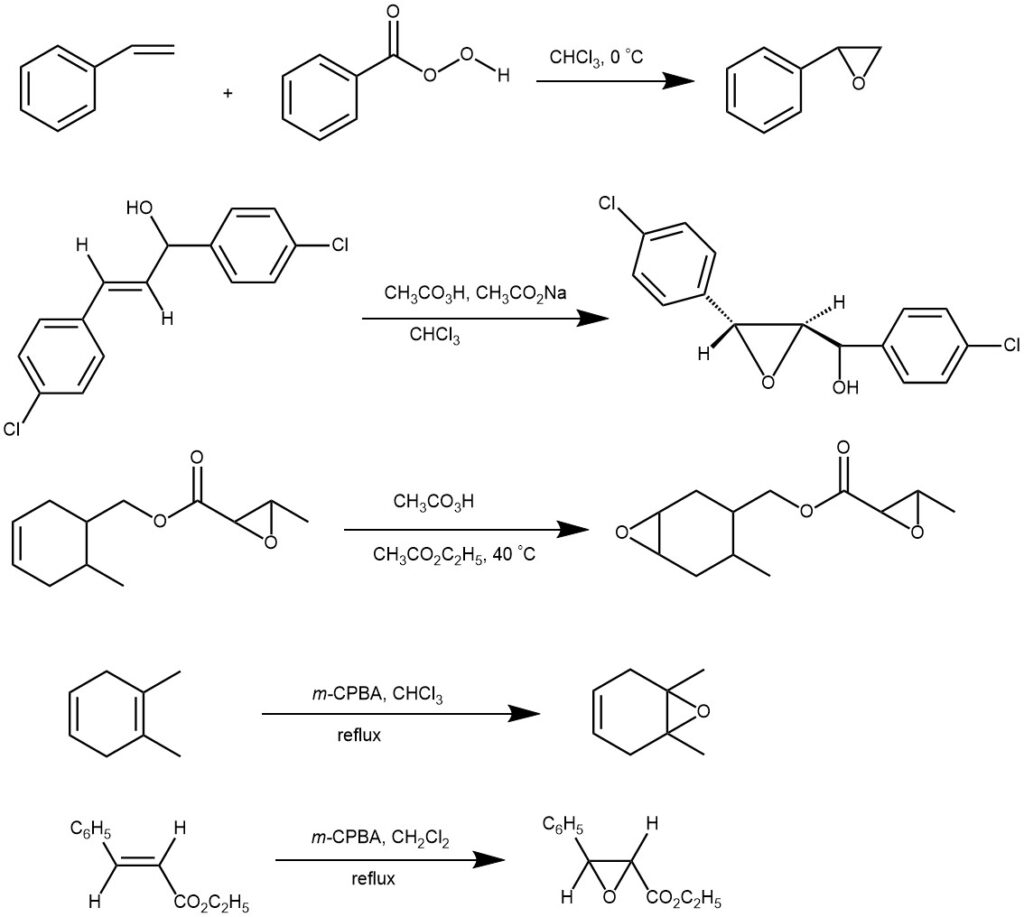
The most widely used peracid for the Prilezhaev reaction is m-CPBA, due to its stability and good solubility in most organic solvents. The reaction is performed in inert solvents (such as hexane, benzene, dichloromethane, chloroform, or carbon tetrachloride) between -10 and 60 °C with the yield of 60-80%.
MECHANISM: The mechanism of the Prilezhaev reaction involves a concerted process in which the peracid acts as an electrophile and the alkene acts as a nucleophile. The oxygen atom of the peracid adds across the double bond of the alkene, forming a cyclic transition state that resembles a butterfly. Peracid usually attacks the olefins from its less hindered side to produce the less hindered epoxide as the major product. At the same time, a proton is transferred from the peracid to the carboxylic acid group, breaking the O-O bond and generating a carboxylic acid as a byproduct.
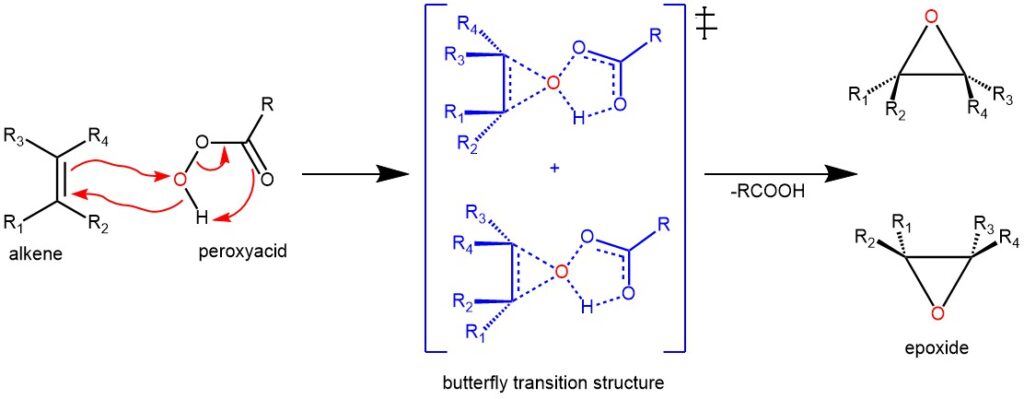
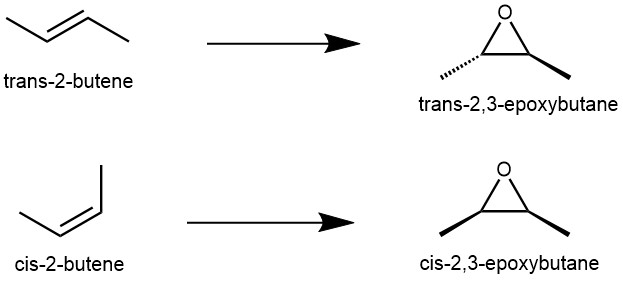
The transition state of the Prilezhaev reaction is highly stereospecific, meaning that the stereochemistry of the alkene is preserved in the epoxide. For example, if we use trans-2-butene as the alkene, we will obtain trans-2,3-epoxybutane as the epoxide. Similarly, if we use cis-2-butene as the alkene, we will obtain cis-2,3-epoxybutane as the epoxide.
APPLICATION: Epoxides made by the Prilezhaev reaction can be further transformed into various functional groups by ring-opening reactions with nucleophiles. For example, they can be converted into diols by hydrolysis with water or acids, into amino alcohols by reaction with ammonia or amines, into ethers by reaction with alcohols or phenols, or into halohydrins by reaction with halides (WILL BE DISCUSSED IN OTHER BLOG POST).
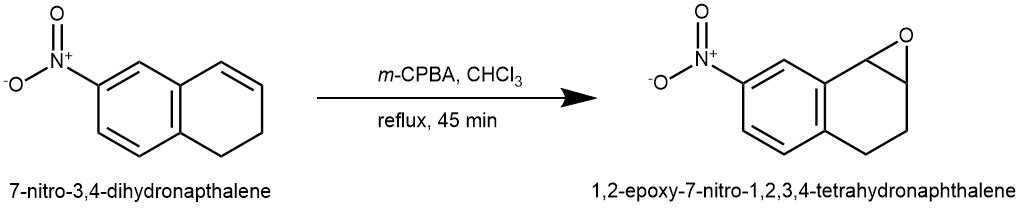
EXAMPLE 1: To a solution of 0.5 g (2.85 mmol) of the 7-nitro-3,4-dihydronapthalene in 9 mL of chloroform was added 0.677 g of m-chloroperoxybenzoic acid in one portion. The resulting solution was heated at reflux for 45 minutes. The mixture was then cooled to 0 0C and the precipitated m-chlorobenzoic acid was removed by filtration. The chloroform layer was evaporated to give a pale-yellow solid which was chromatographed over silica gel (50 g) and eluted with dichloromethane. The relevant fractions were combined and evaporated to give 0.487 g (89.5%) of l,2-E poxy-7-nitro-l,2,3,4-tetrahydronapthalene (REF: World Intellectual Property Organization WO 98/12181).
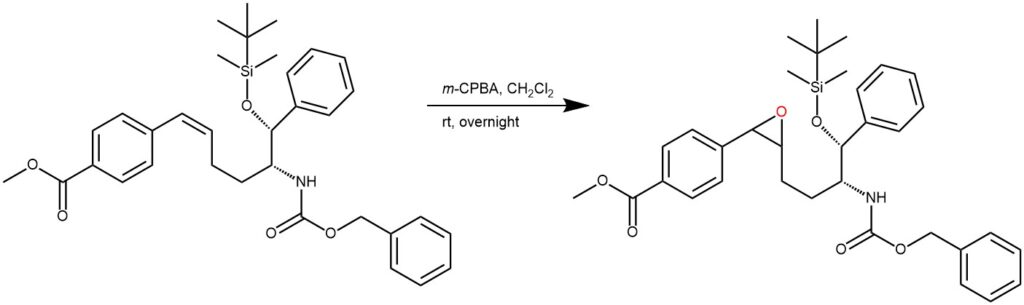
EXAMPLE 2: To a solution of the starting material (0.880 g, 1.85 mmol) in dichloromethane (20 ml) was added 3-chloroperbenzoic acid (0.60 g, 2.0 mmol) in portions. The reaction mixture was stirred at ambient temperature overnight and it was then washed with sodium carbonate and dried over magnesium sulfate. After concentration, the residue was purified by flash column chromatography (0-70% ethyl acetate in hexanes) and 0.90 g (100%) of the title compound was obtained as mixtureof diastereomers (REF: World Intellectual Property Organizaton WO2011025690)
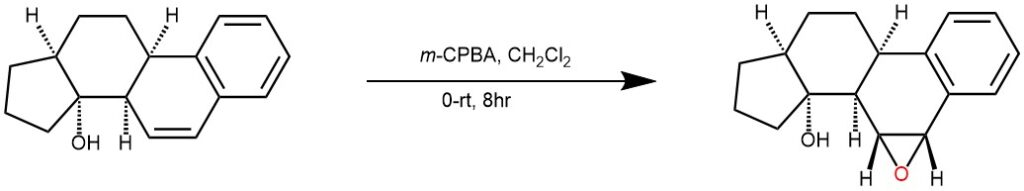
EXAMPLE 3: m-chloroperbenzoic acid (70%, 1.05 mmol) was added to a solution of rac-(3aR,3bR,9bR,11aS)-1,2,3,3b,9b,10,11,11a-octahydro-3aH-cyclopenta[α]phenanthren-3a-ol (1.00 mmol) in CH2Cl2 (1.5 mL) and stirred the mixture at 0 °C for 30 minutes then at room temperature. After 8 hours, the solution was diluted with CH2Cl2(10 mL) and washed the solution with NaOH (4N) and then with distilled water. The organic layer was separated. And dried the organic layer over MgSO4, filtered and solvent evaportated to get the desired product as colorless solid (REF: Chemistry – A European Journal (2007), 13(21), 6047-6062.)
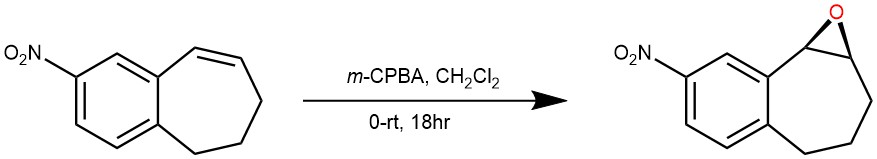
EXAMPLE 4: To a cold (0° C.) solution of the starting material (0.539 g, 2.85 mmol) in methylene chloride (10 ml) was added 75% meta-chloroperoxybenzoic acid (0.79 g, 4.56 mmol). The reaction mixture was allowed to come to room temperature over an 18 hour period. The reaction mixture was diluted with methylene chloride and washed with saturated potassium hydrogen carbonate solution. Dried over MgSO4, filtered and evaporated to give the epoxide as a yellow solid (0.547 g, 94% yield) )[REF: United States Patents US 6,333,349 B l]
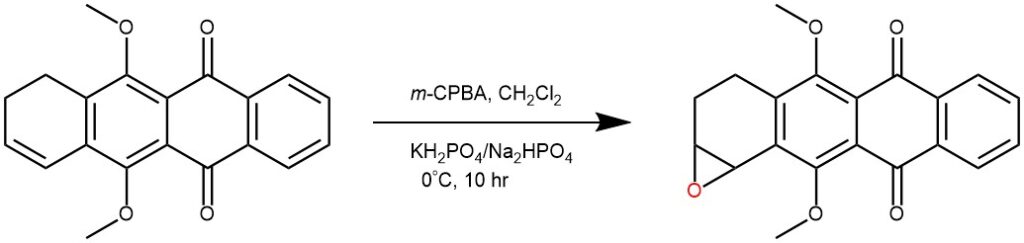
EXAMPLE 5: To a mixture containing starting material (1.6 g, 5 mmol), CH2Cl2 (75 mL) and buffer solution (75 mL) (KH2PO4/Na2HPO4) is added 3- chloroperoxybenzoic acid (mCPBA) (0.8 g) and the resulting mixture then stirred at 0 °C for 10 h. The organic layer is separated, washed successively with saturated aqueous NaHCO3 (2 x 50 mL), 3% aqueous NaHSO4 (50 mL) and brine (50 mL), then dried (Na2SO4). Evaporation of the solvent (rotary evaporator) afforded epoxide as yellow solid, 1.68 g yield 99% [REF: Synthesis (1991), (1), 33-6)].
REFERENCES:
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti, Barbara Czako
- Modern synthetic reactions by Herbert O. House


