Parikh-Doering Oxidation:
The oxidation of primary and secondary alcohol to aldehyde and ketone respectively using sulfur trioxide in the form of its pyridine complex (SO3.C5H5N) and dimethyl sulfoxide (DMSO) in the presence of a base [triethyl amine (TEA)] is called as the Parikh-Doering oxidation. In this reaction, DMSO acts as an oxidant, and SO3 as the activator. Dichloromethane (DCM) is the solvent of choice.
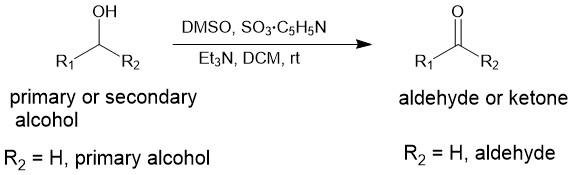
The oxidation is, typically, carried out by the addition of a solution of pyridine-sulfur trioxide (SO3.C5H5N) complex in DMSO to a mixture of the alcohol, DMSO, and TEA at 25 °C. The oxidation is very rapid at room temperature and reaches completion usually within minutes
MECHANISM:
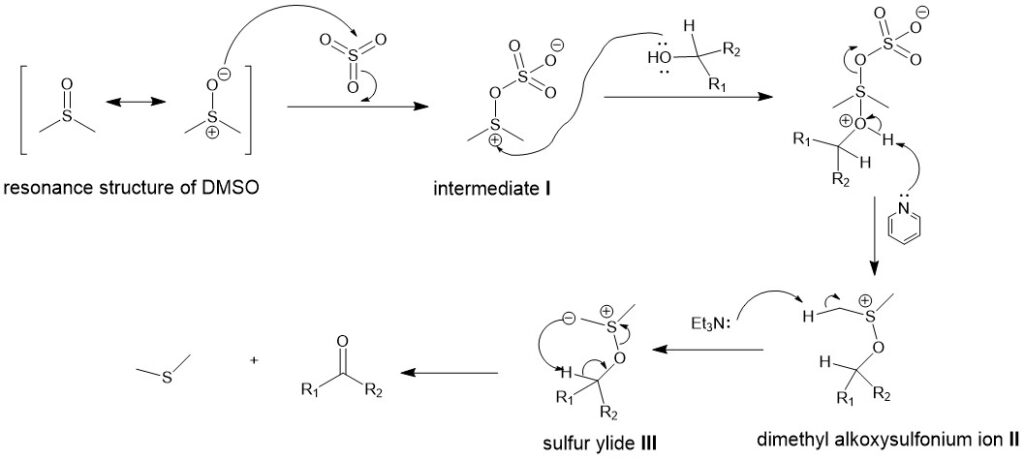
Mechanistically, it involves the activation of DMSO by SO3 to form an intermediate (I). Nucleophilic attack by alcohol followed by proton transfer to base (triethyl amine or pyridine) forms alkoxysulfonium ion (II). Deprotonation by triethyl amine forms the sulfur ylide (III) which through a five-membered transition state affords the carbonyl compound and the dimethyl sulfide.
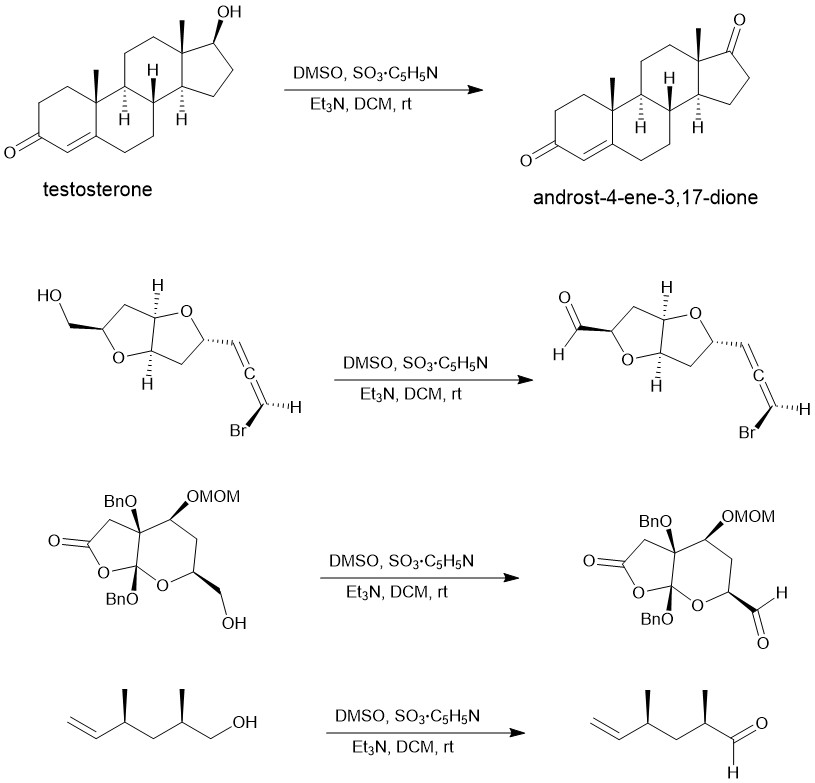
Eg.
SWERN OXIDATION USING TRIFLUOROACETIC ANHYDRIDE (TFAA):
In 1976, D. Swern and co-workers reported that treatment of dimethyl sulfoxide (DMSO) with below trifluoroacetic anhydride (TFAA) -50 °C in dichloromethane (DCM) first resulted in an intermediate called trifluoroacetoxydimethylsulfonium trifluoroacetate, which reacted efficiently with primary and secondary alcohols forming yet another intermediate called alkoxydimethylsulfonium trifluoroacetates. This intermediate upon the addition of triethyl amine (TEA) afforded corresponding aldehydes or ketones. In simple words, dimethyl sulfoxide-trifluoroacetic anhydride converts primary and secondary alcohols to the corresponding aldehydes or ketones in the presence of a base (eg. Triethyl amine).
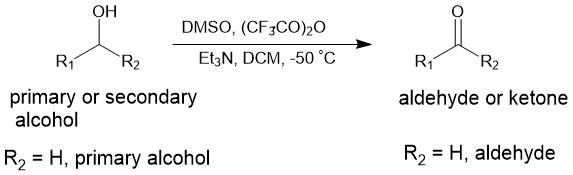
It should be noted that DMSO reacts violently with TFAA at room temperature. However, the reaction can be controlled at low temperatures and the intermediate is stable at or below -30 °C. Typically, TFAA in DCM is added dropwise to a cold (< -50 °C), stirring solution of DMSO in DCM whereupon white precipitate forms. A solution of alcohol in DCM is then added dropwise to the mixture followed by the addition of TEA after about 30 minutes.
MECHANISM:
The mechanism is similar to that of Parikh-Doering oxidation. The first step involves activation of DMSO with TFAA to form the trifluoroacetoxydimethylsulfonium trifluoroacetate intermediate to which a nucleophilic attack by the alcohol and abstraction of proton by the base (triethyl amine) forms the sulfur ylide. The ylide then decomposes to form the aldehyde or ketone and the dimethyl sulfide.
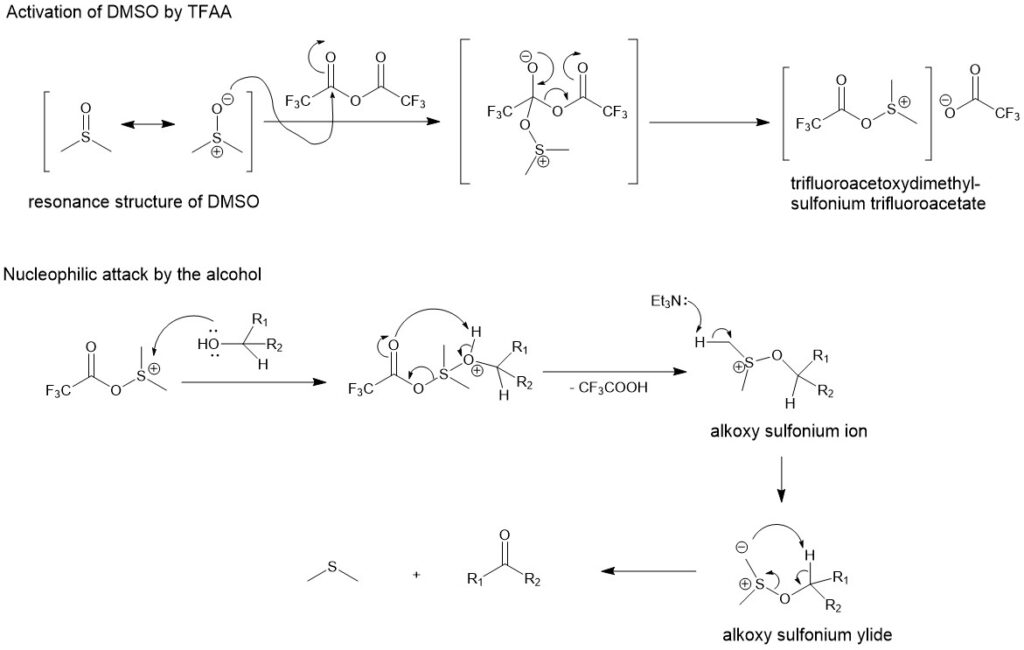
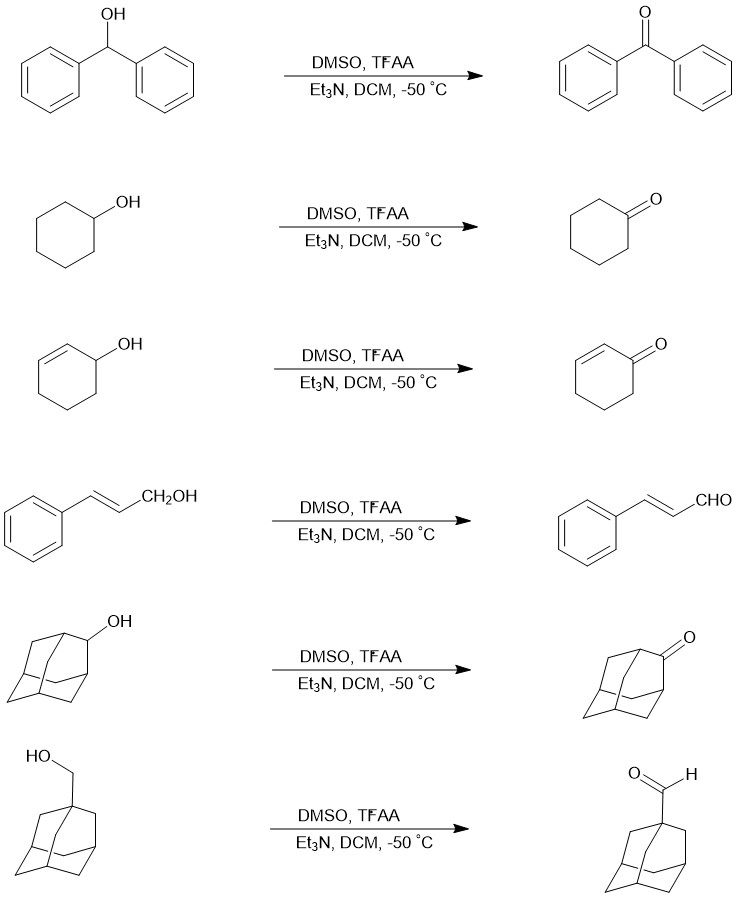
Eg.
SWERN OXIDATION USING OXALYL CHLORIDE:
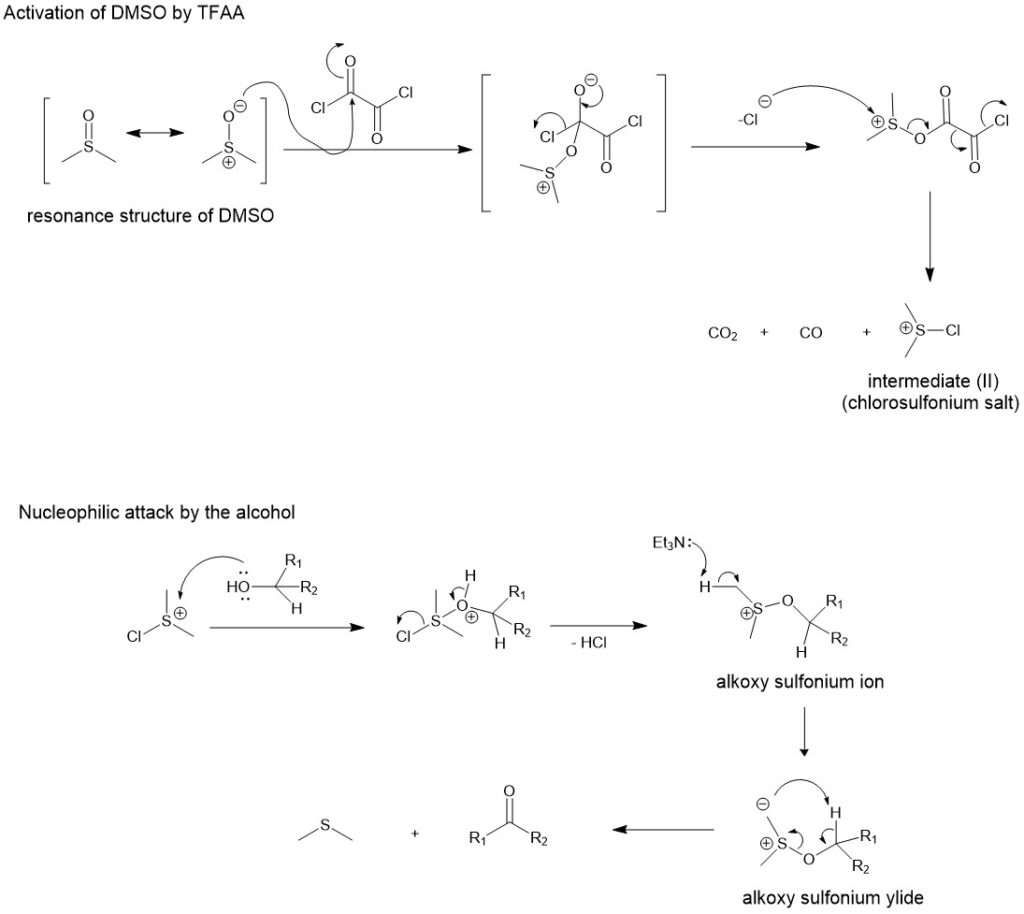
In 1978, oxalyl chloride was found to be more efficient than TFAA as an activating agent for DMSO in alcohol oxidation. Thus oxidation of primary and secondary alcohol using DMSO as the oxidant and oxalyl chloride as the activator in presence of a base is referred to as Swern oxidation. For this reaction, the initial intermediate is unstable above -60 °. So, the reaction is usually conducted at -78 °C.
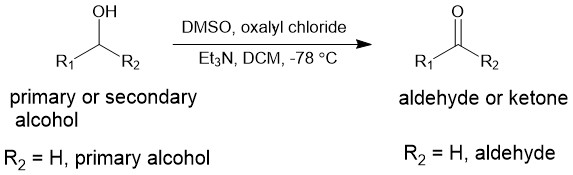
Similar to the reaction of DMSO with TFAA, oxalyl chloride also reacts violently and exothermically with DMSO at room temperature. Therefore, a low temperature of -60 °C or lower is used for the formation of intermediate (I) which loses carbon dioxide and carbon monoxide to form intermediate (II).
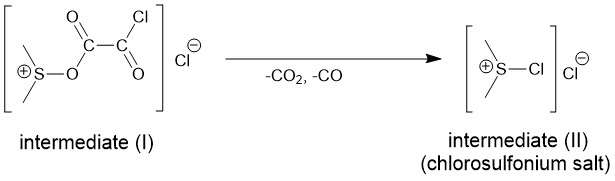
Typically, DMSO is added to the stirred oxalyl chloride solution in DCM at -60 °C. The mixture is stirred for about 2 minutes followed by the addition of the solution of alcohol also in DCM and the triethyl amine is added lastly. The mixture is then warmed to room temperature.
MECHANISM:
The mechanism is very much similar to that of the DMSO and TFAA reactions.
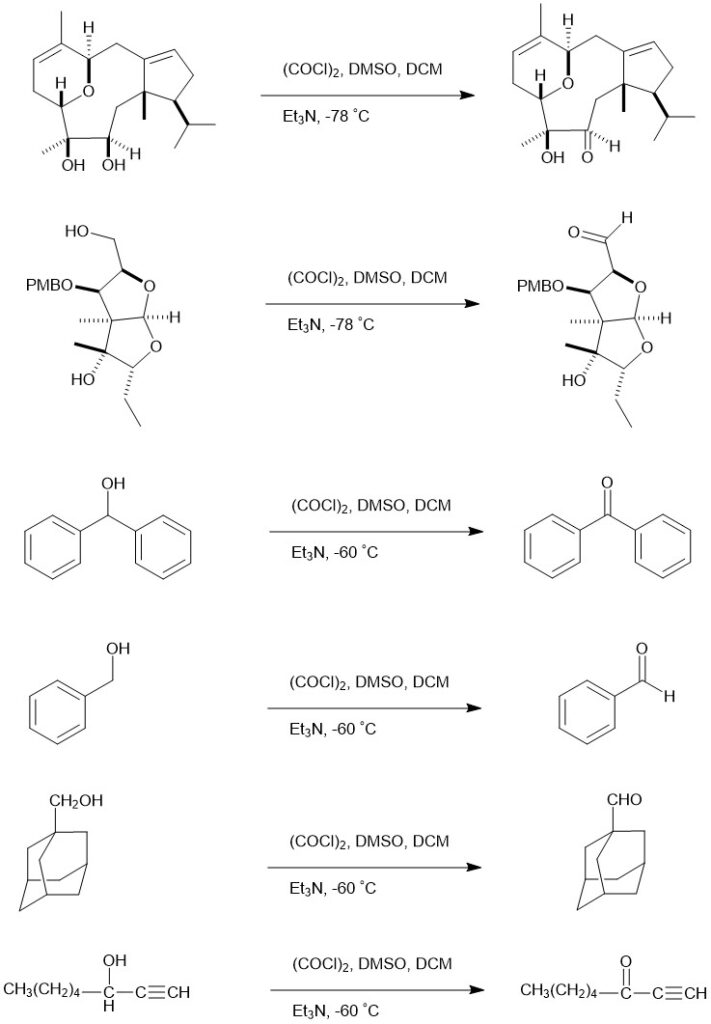
Eg.
References
- J. Am. Chem. Soc., 1967, 89, 5505
- J. Org. Chem., 1976, 41, 3329
- J. Org. Chem., 1976, 41, 957
- Tetrahedron 1978, 34, 1651
- J. Org. Chem., 1979, 44, 4148
- J. Org. Chem., 1978, 43, 2480