Inorganic chemists visualize oxidation reactions either as a loss of electrons or an increase in the oxidation number. However, it is difficult to visualize an organic reaction under these terms. It is more convenient to see an organic compound being oxidized as a gain of oxygen or a loss of hydrogen. In the case of alcohols, primary alcohols are oxidized first to aldehydes which can be further oxidized to carboxylic acids. Secondary alcohols are oxidized to ketones. The ketones can be oxidized further, however, only at the expense of the C-C bond dissociation.
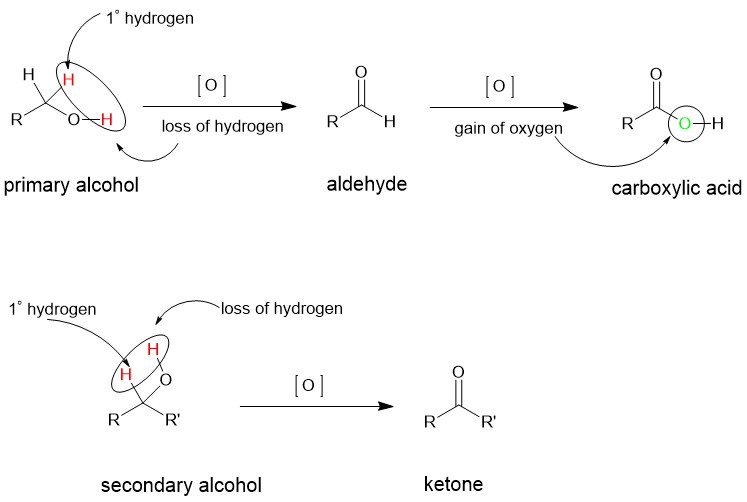
It should be noted that it is one of the primary hydrogens which is lost in alcohol during the oxidation process. If the alcohol does not have any primary hydrogen then it cannot be oxidized. It is, for this reason, tertiary alcohols are resistant to oxidation reactions.
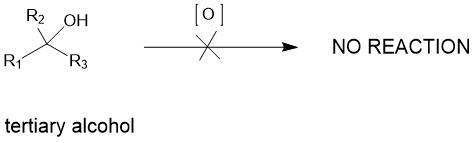
JONES REAGENT:
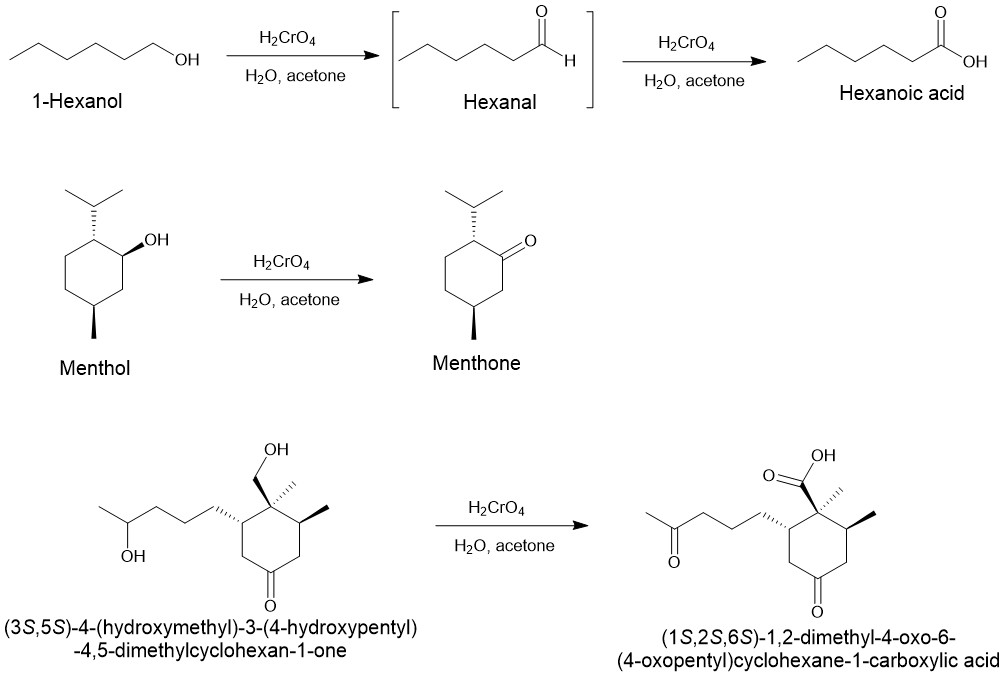
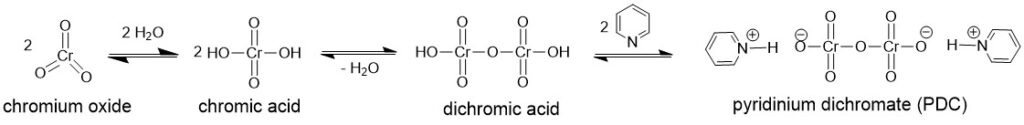
It is a mixture of chromium (VI) oxide or sodium or potassium dichromate in aqueous sulfuric acid, which forms chromic acid in situ. Thus, a solution of chromic acid in aqueous sulfuric acid is known as the Jones reagent. Since most alcohols have poor solubility in water, oxidation with the Jones reagent is carried out by dissolving the alcohols in acetone.
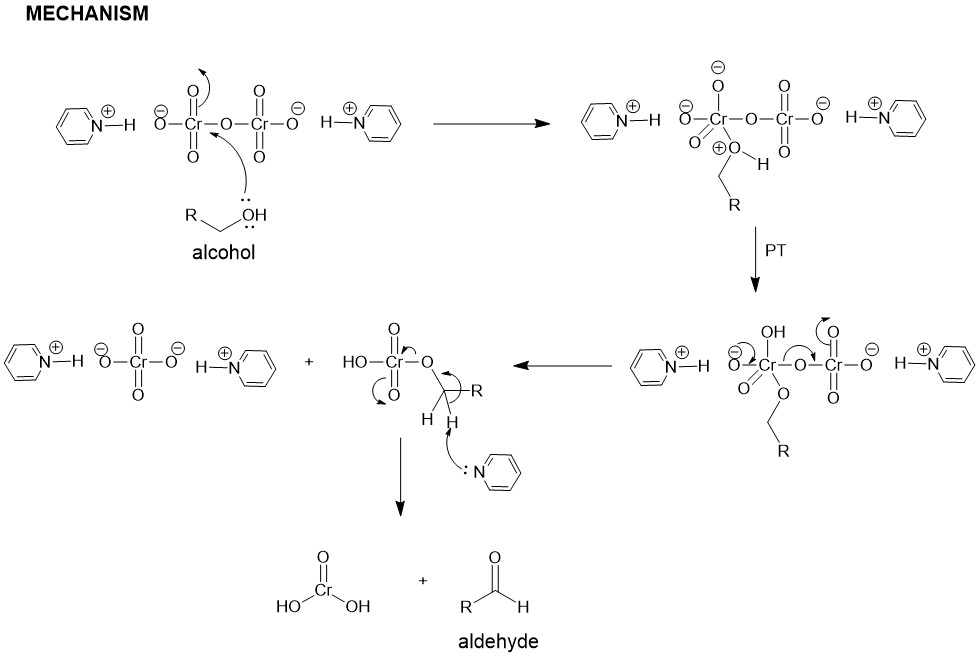
The alcohol reacts with the chromic acid, in a mechanism similar to the Fischer Esterification, to give an alkyl chromate ester which either intramolecularly or intermolecularly in the presence of a base ( water) yields the carbonyl compound.
MECHANISM:
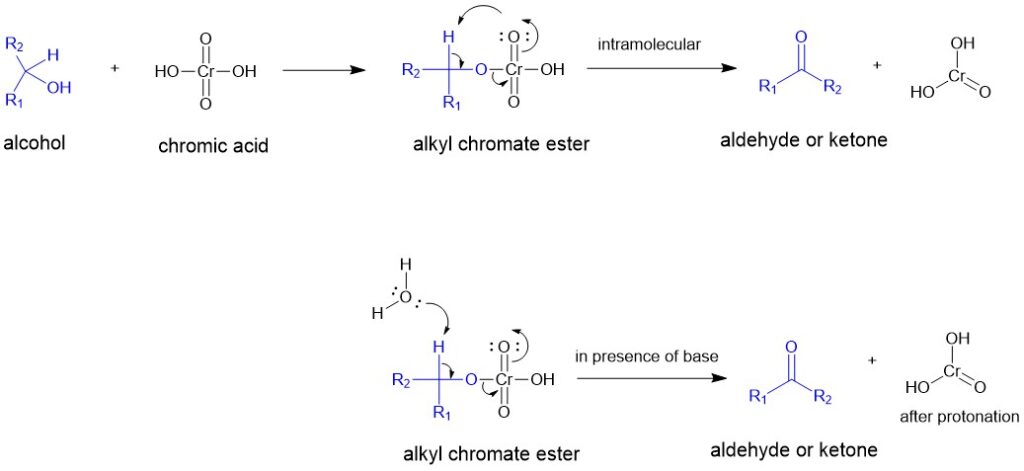
In most cases, the primary alcohol is oxidized to aldehyde which is oxidized further to form a carboxylic acid. Aldehydes that can form hydrates in the presence of water are further oxidized to carboxylic acid.
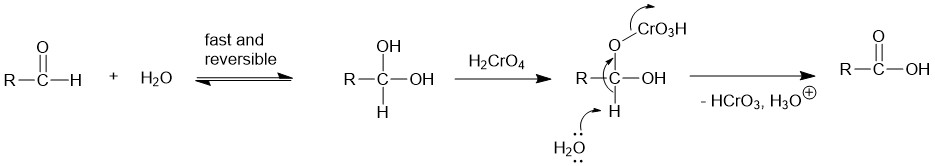
Eg.
COLLIN’S REAGENT:
It is a red-colored complex of chromium (VI) oxide with pyridine in dichloromethane (DCM). It is generally obtained by adding 1 equivalent of chromium (VI) oxide to a solution of 2 equivalent of pyridine in DCM to initially precipitate as an intractable, yellow microcrystalline which on continuous stirring at 15 °C formed deep-red crystals which could be isolated, dried and stored with ease for the reaction. Because of the extreme ease of hydration, precautions must be taken to limit exposure of the complex to moisture during isolation or use.
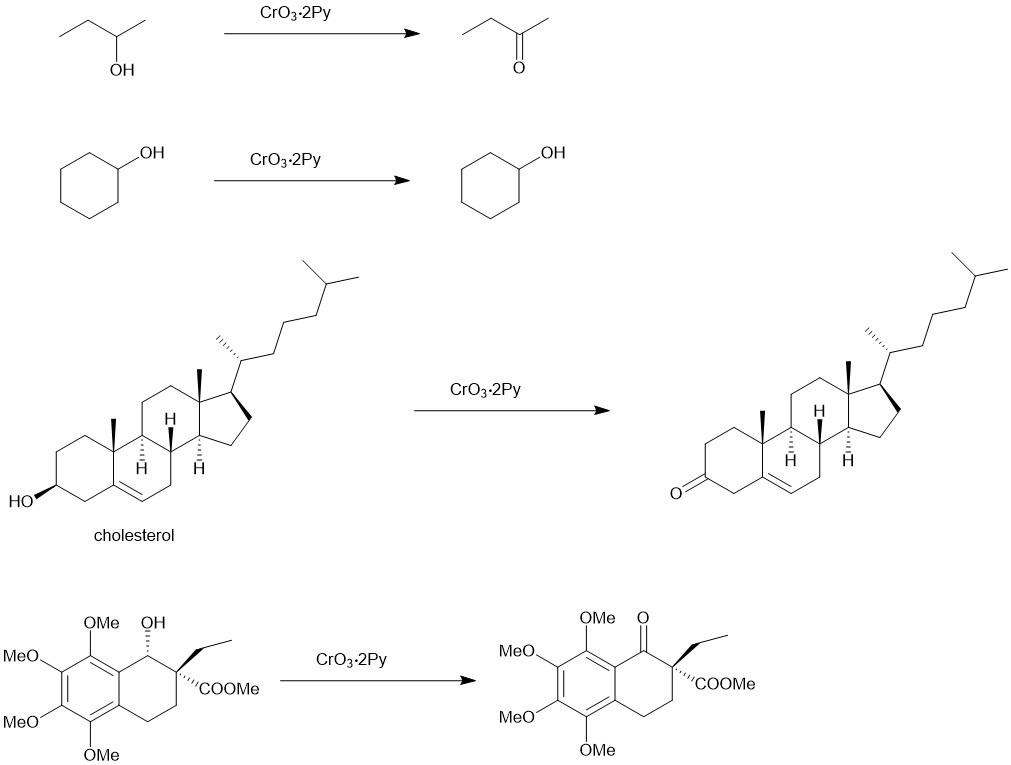
Since this reagent does not contain water, it is especially useful for the oxidation of primary alcohols to aldehydes preventing further oxidation of the aldehyde to carboxylic acid. The secondary alcohols are oxidized to ketones.
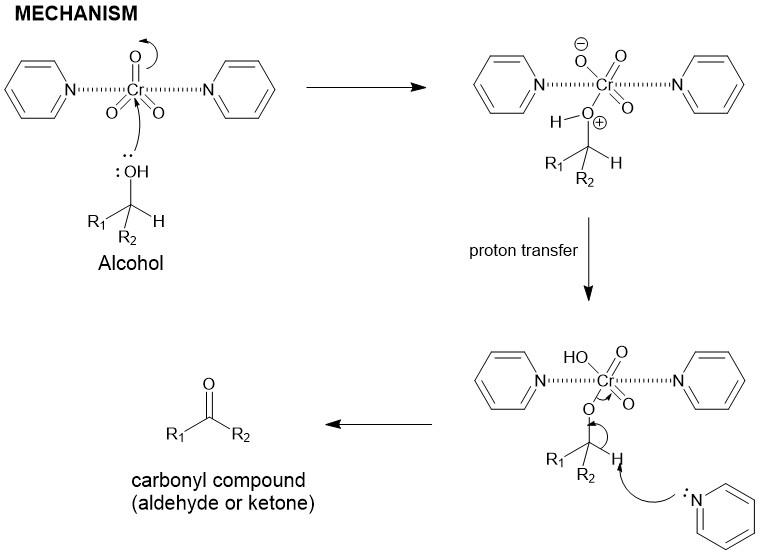
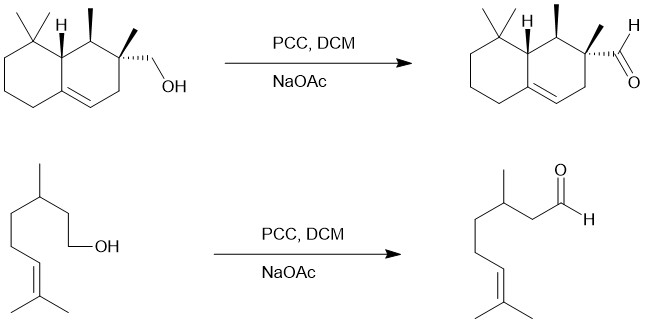
PYRIDINIUM CHLOROCHROMATE (PCC) OR COREY-SUGGS REAGENT:
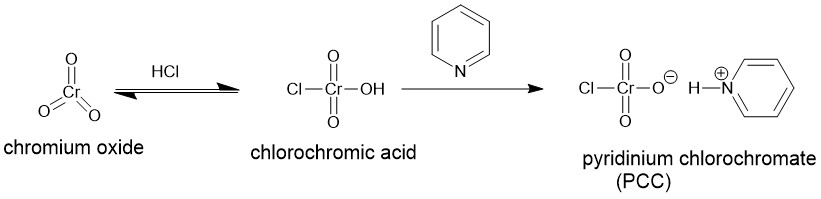
It is yet another chromium (VI) based reagent for the oxidation of alcohols to carbonyl compounds. It is, generally, prepared by the addition of pyridine to a solution of chromium trioxide (CrO3) in 6M HCl. Filtration of the mixture affords (PCC), a yellow-orange solid.
For a small-scale experiment, PCC is suspended in dichloromethane (PCC) and the alcohol is rapidly added at room temperature. DCM is a superior solvent for the conversion of a primary alcohol to an aldehyde. The aldehyde is not oxidized further to a carboxylic acid. However, if water is present, it can add to the aldehyde to create the aldehyde hydrate which could be further oxidized by a second equivalent of PCC. Secondary alcohol straightforwardly gives ketone.
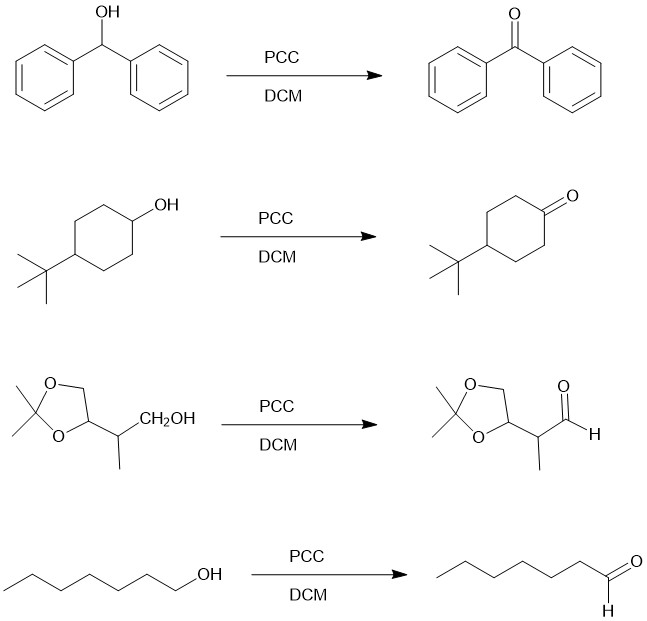
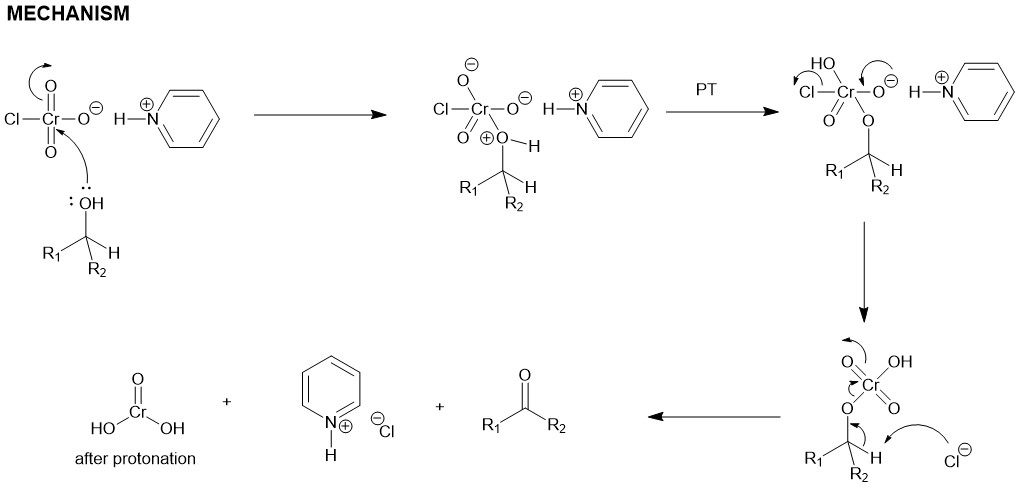
PCC is slightly acidic, so acid-labile compounds can only be oxidized by buffering the reaction mixture with powdered sodium acetate. If DMF is used instead of DCM, primary alcohol is oxidized further to give carboxylic acids.
PYRIDINIUM DICHROMATE (PDC) OR COREY-SCHMIDT REAGENT:
So far we have seen different chromium (VI) based reagents for the oxidation of alcohols to carbonyl compounds. Jones reagent causes over-oxidation of a primary alcohol to carboxylic acid, Collins reagent is difficult to prepare and handle and PCC is not suitable for acid-labile substrates and products. Non-aqueous Pyridinium dichromate (PDC) emerged as a very useful and versatile oxidant for the oxidation of alcohol to carbonyl compounds. PDC is a stable, bright orange-colored solid prepared by dissolving CrO3 in a minimum quantity of water followed by the addition of pyridine and collecting the precipitate. PDC is very soluble in water, dimethylformamide (DMF), dimethylsulfoxide (DMSO), and dimethylacetamide (DMAC) while being only partially soluble in dichloromethane (DCM), chloroform, and acetone. It is unstable in acetonitrile.
Pyridinium dichromate is less acidic than PCC hence it is more suitable for the oxidation of acid-labile substrates. The oxidation product also depends on the solvent used. In DCM, it conveniently oxidizes primary alcohol to aldehyde and secondary alcohol to ketones. However, in DMF, the non-conjugated saturated primary alcohols are oxidized to carboxylic acids but allylic and are oxidized only to aldehydes. The secondary alcohols are oxidized benzylic alcohols to ketones.
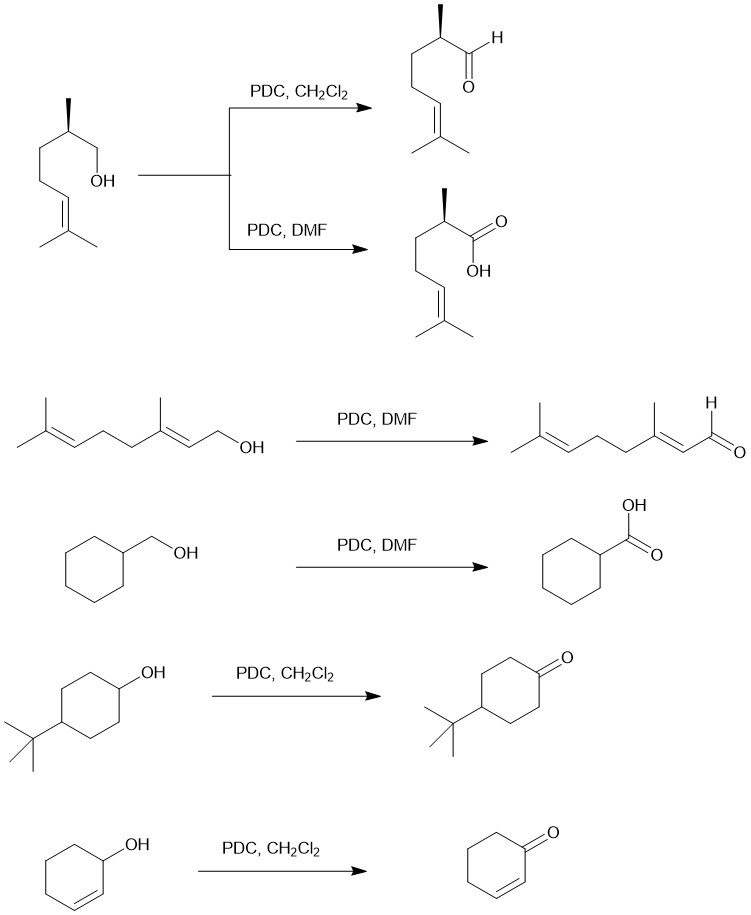
The following guidelines can help in the election of a certain chromium-based oxidant in the laboratory:
- Jones oxidation is very easy to carry out, because of the absence of the need to keep anhydrous conditions. Furthermore, it is very cheap. It is the oxidation of choice for robust substrates on a big scale. It is neither suitable for very acid-sensitive substrates nor for the preparation of many aldehydes.
- Collins oxidation is very cheap but has the added experimental difficulty of having to work under anhydrous conditions. Although sometimes it lacks the selectivity of PDC or PCC, it can produce very good yields of aldehydes and ketones in uncomplicated substrates.
- PDC and PCC are more expensive reagents that normally guarantee the best results in difficult cases.



