OXIDATION OF ALCOHOL: Potassium permanganate (KMnO4) is a very strong oxidant able to oxidize many functional groups like alcohols, aldehydes, alkenes, etc. Under controlled conditions, KMnO4 oxidizes primary alcohols to carboxylic acids and secondary alcohols to ketones.
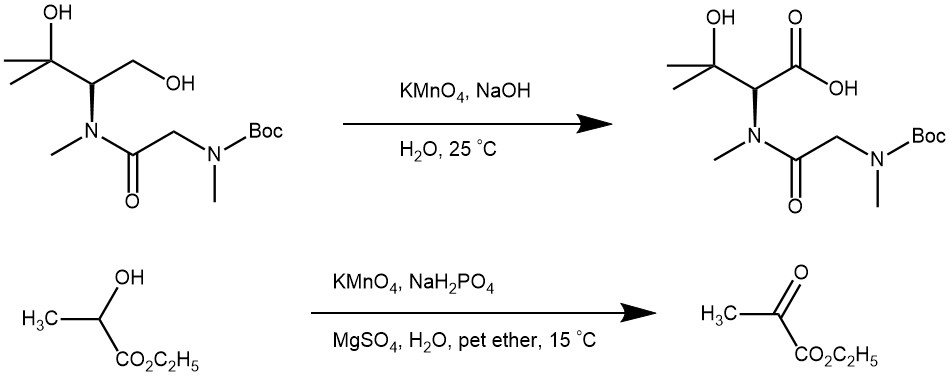
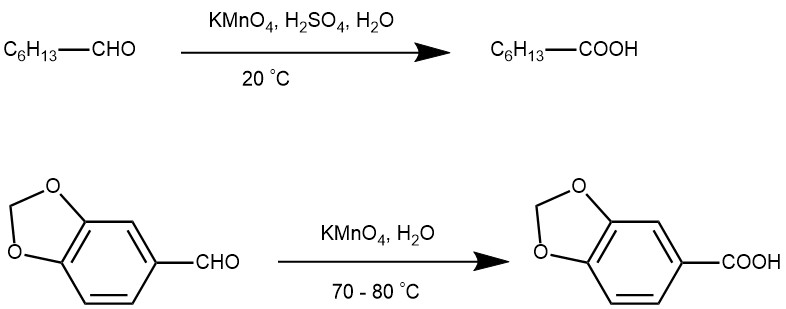
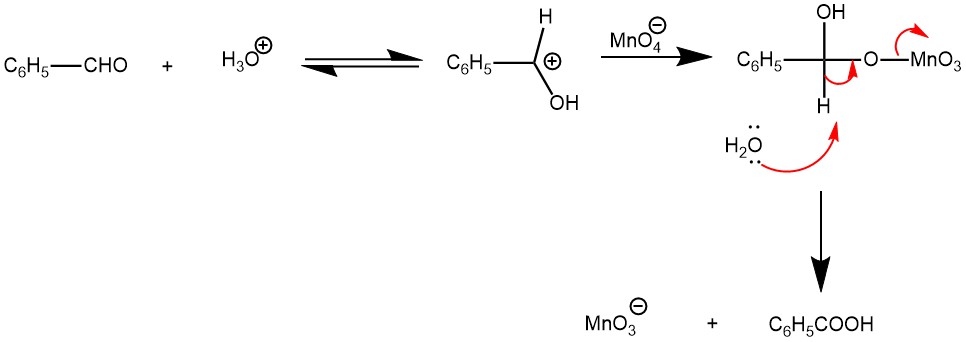
OXIDATION OF ALDEHYDE: Aqueous solution of potassium permanganate (KMnO4) under acidic or basic conditions is a more commonly employed reagent for the oxidation of the aldehyde to carboxylic acid.
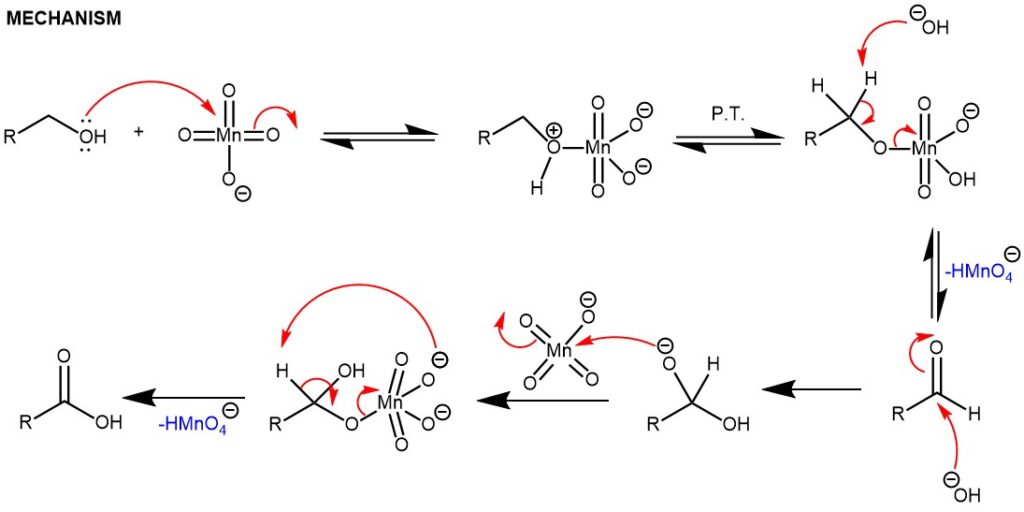
In an acidic medium, the reaction is accelerated by the presence of electron-donating groups and is believed to involve the formation and subsequent decomposition of the permanganate ester.
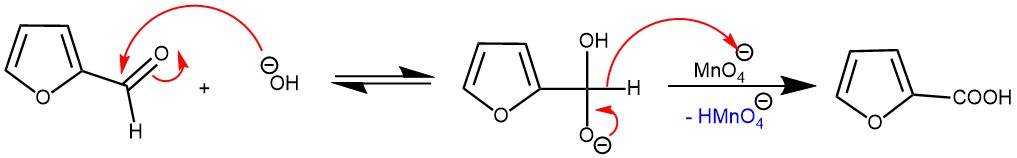
On the other hand, oxidation of the aldehyde in a basic medium is accelerated by electron-withdrawing groups and proceeds by a hydride transfer mechanism. The reaction also requires an acidic workup since the carboxylic acid is deprotonated under basic conditions.
OXIDATION OF CARBON-CARBON DOUBLE BONDS: In an alkaline medium, an aqueous solution of potassium permanganate reacts with an alkene to add two hydroxyl functions to the double bond in a cis manner.
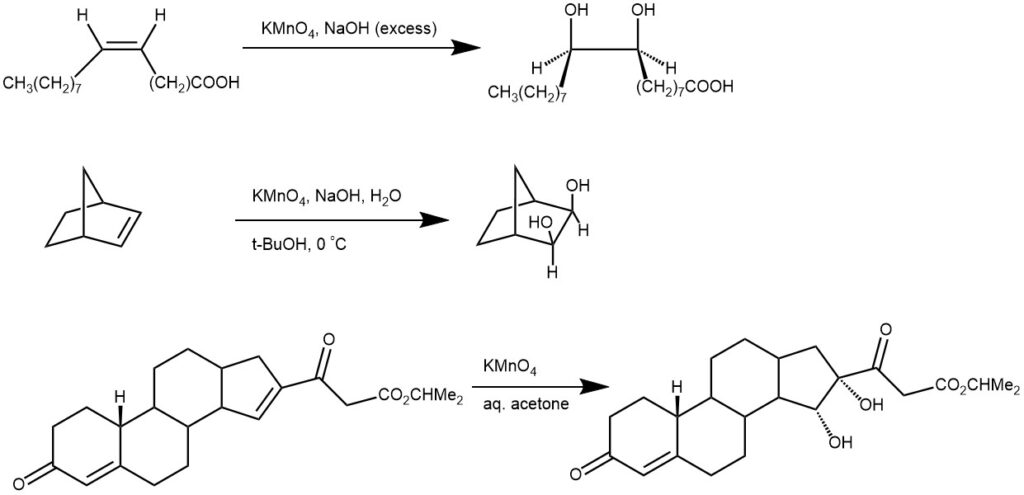
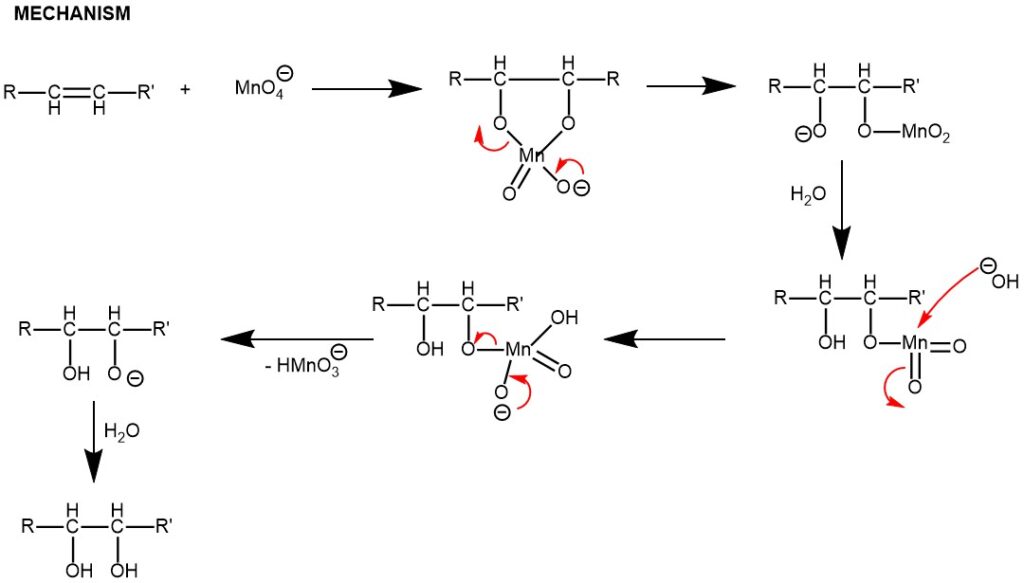
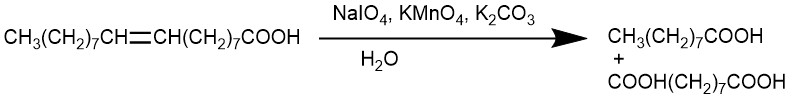
An important modification of the permanganate hydroxylation procedure utilizes a mixture of potassium permanganate and potassium periodate in an aqueous solution at PH 7.7 for the oxidation of alkene. The C-C bond cleavage forms the carbonyl compounds. Any aldehydes produced by this cleavage are oxidized to carboxylic acids.
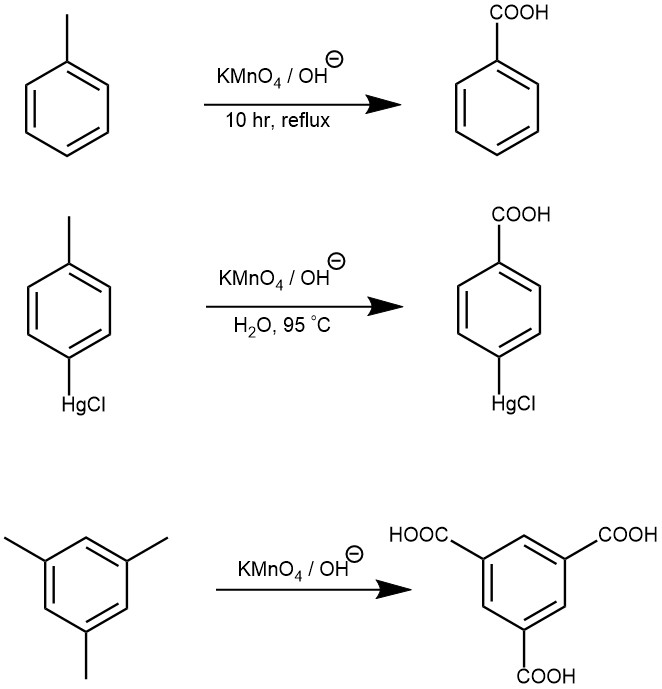
OXIDATION OF CARBON-HYDROGEN BONDS: Oxidation of the side chain of benzene derivatives with an aqueous alkaline KMnO4 gives the carboxylic acid.