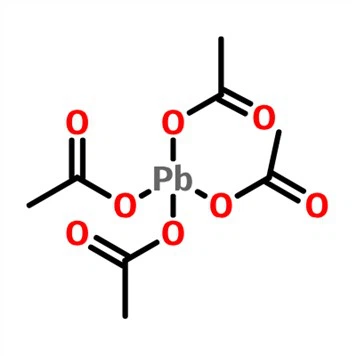
Lead tetraacetate [Pb(OAc)4] is a commercially available crystalline solid used as a solution in acetic acid or benzene for the oxidation of organic compounds. Although lead tetraacetate may be employed for the oxidation of wide variety of organic compounds, its primary use is for the oxidative cleavage of 1,2-diols. Oxidative cleavage of diols, particularly vicinal diols, is an important organic reaction that has been extensively studied over the years. One of the most widely used reagents for this reaction is lead tetraacetate [Pb(OAc)4]. The reaction is also known as the Criegee glycol oxidation, named after the German chemist Rudolf Criegee, who first reported the reaction in 1953.
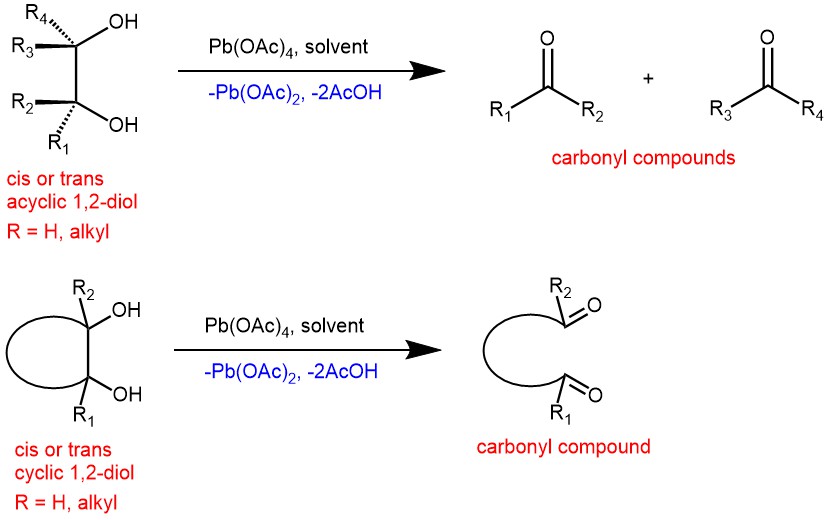
EXAMPLES:
MECHANISM: The preferred pathways for the cleavage of 1,2-diols seems to involve formation of a five-membered cyclic intermediate which decomposes to give carbonyl compounds.
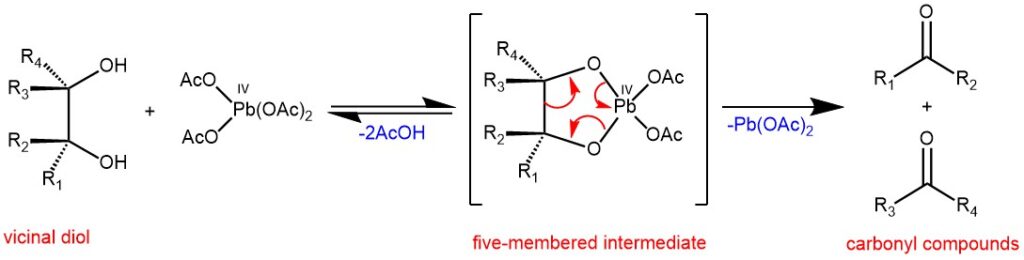
Cis-vicinal diols and threo-diols are cleaved much faster than the corresponding trans-vicinal diols and erythro-diols.
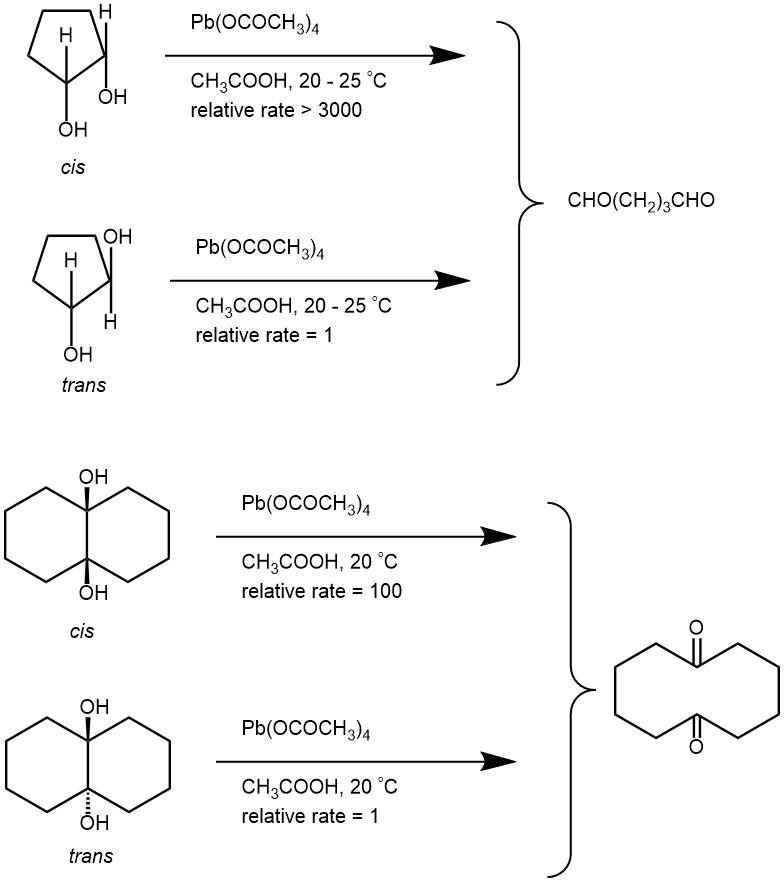
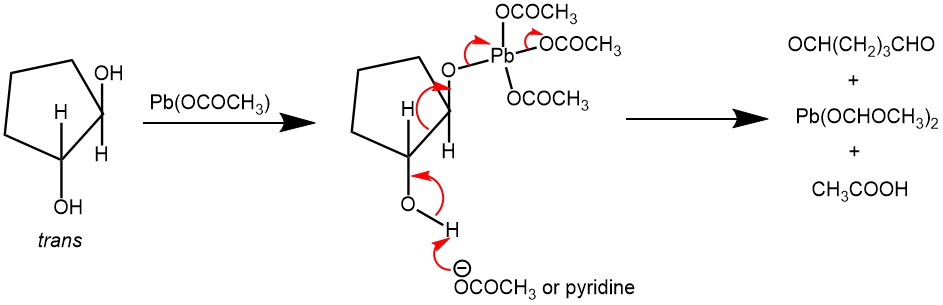
As cyclic intermediate was not possible with trans-1,2-diols, it was noted that the cleavage of trans-1,2-diols was very slow with Pb(OAc)4 in acetic acid. However, the cleavage of trans-1,2-diol is faster if pyridine was used as the solvent. A second mechanism, as presented below, is proposed for sterically unfavorable diols (eg. trans -1,2-diol) which does not require a cyclic intermediate.
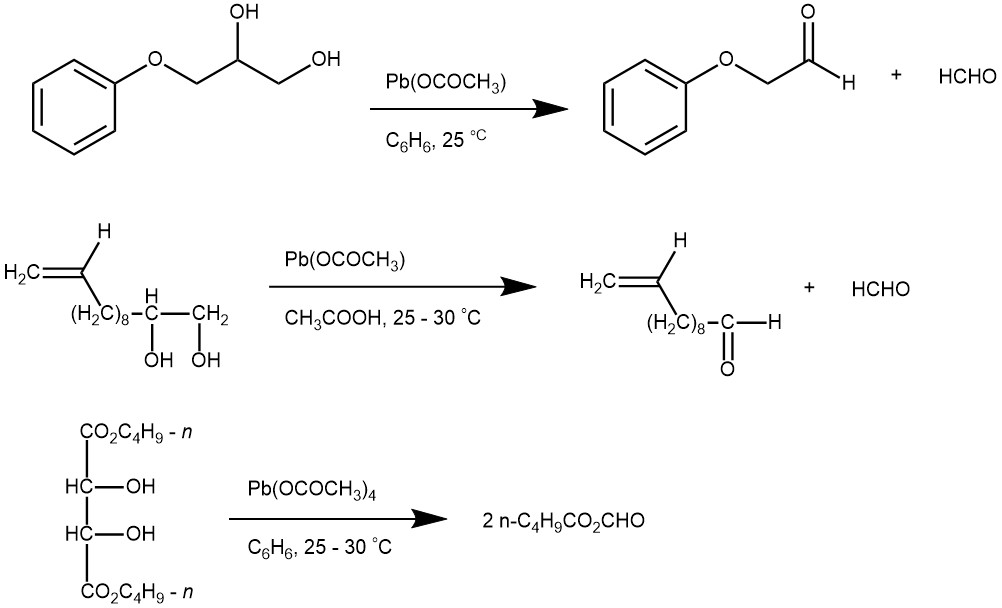
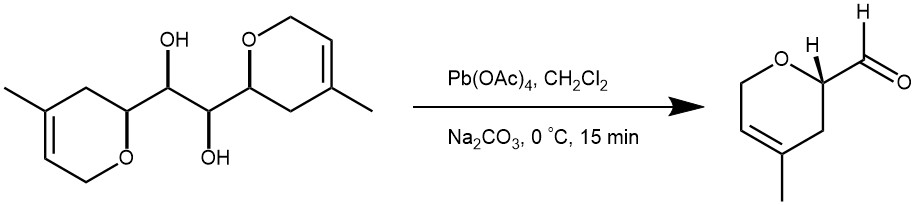
EXAMPLE 1: A solution of Pb(OAc)4 (177 mg, 0.4 mmol) in CH2Cl2 (5 mL) was added dropwise to a suspension of the diol (50 mg, 0.20 mmol) and solid Na2CO3 (310 mg, 2.4 mmol) in CH2Cl2 (5 mL) at 0 0C. After 15 minutes, TLC showed complete consumption of starting material. The mixture was diluted with Et2O, filtered through a short pad of celite and carefully concentrated at room temperature under vacuum. This led to highly volatile aldehyde product (0.40 mmol). [Synthesis 2001, 13, 2007-2010]
Reference:
- Modern Synthetic Reactions by Herbert O. House
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako