In the previous article we have discussed about the deoxygenation of alcohols, which is a common synthetic procedure. There are two main methods for deoxygenation: radical and nucleophilic. The radical deoxygenation method was exemplified by the Barton-McCombie reaction, which involves the free-radical reduction of a thiocarbonyl derivative of the alcohol (for more details see our previous blog). This method is mild, efficient, and compatible with a wide range of functional groups. It is particularly suitable for secondary alcohols but has also been applied to tertiary and primary alcohols.
Nucleophilic deoxygenations, on the other hand, employ metal hydride reagents, such as lithium aluminum hydride or lithium triethylborohydride, to reduce an activated derivative of the alcohol, such as a halide or sulfonate ester. Unlike the radical method, nucleophilic deoxygenation is most efficient for unhindered primary alcohols. However, a main limitation of nucleophilic deoxygenation is that it is incompatible with reducible functional groups. The important thing is that the alcohol has to be derivatized to some other functional group which is an activator of the alcohol or a good leaving group.
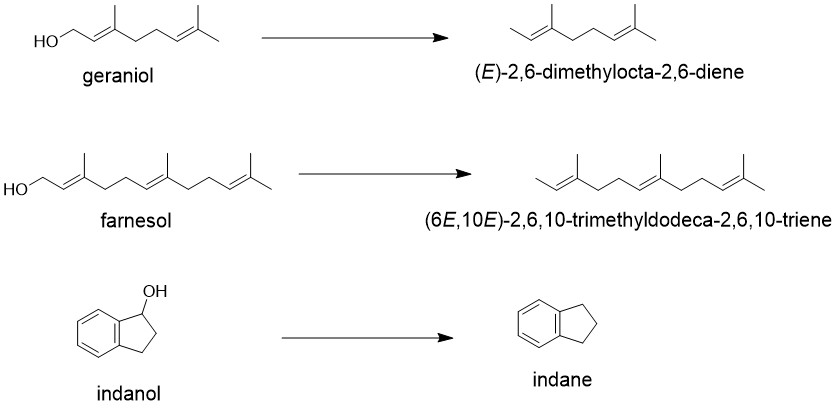
One of the earliest method of the deoxygenation was developed by E. J. Corey and Achiwa in 1969. Their solution involved using a pyridine-sulfur trioxide complex in tetrahydrofuran as the reagent for hydroxyl activation and carrying out the reduction step with Lithium Aluminum Hydride in tetrahydrofuran without isolating the intermediate sulfate monoester.
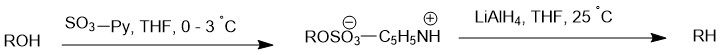
In a typical procedure for deoxygenating an alcohol using a pyridine-sulfur trioxide complex in tetrahydrofuran as the reagent for hydroxyl activation and lithium aluminum hydride in tetrahydrofuran for reduction, the alcohol was first converted to the sulfate monoester in tetrahydrofuran at 0-3°C using a moderate excess of the pyridine-sulfur trioxide reagent. Thin layer chromatographic (TLC) analysis was used to monitor the progress of the reaction over time. After complete esterification, an excess of lithium aluminum hydride in tetrahydrofuran was added to effect reduction.
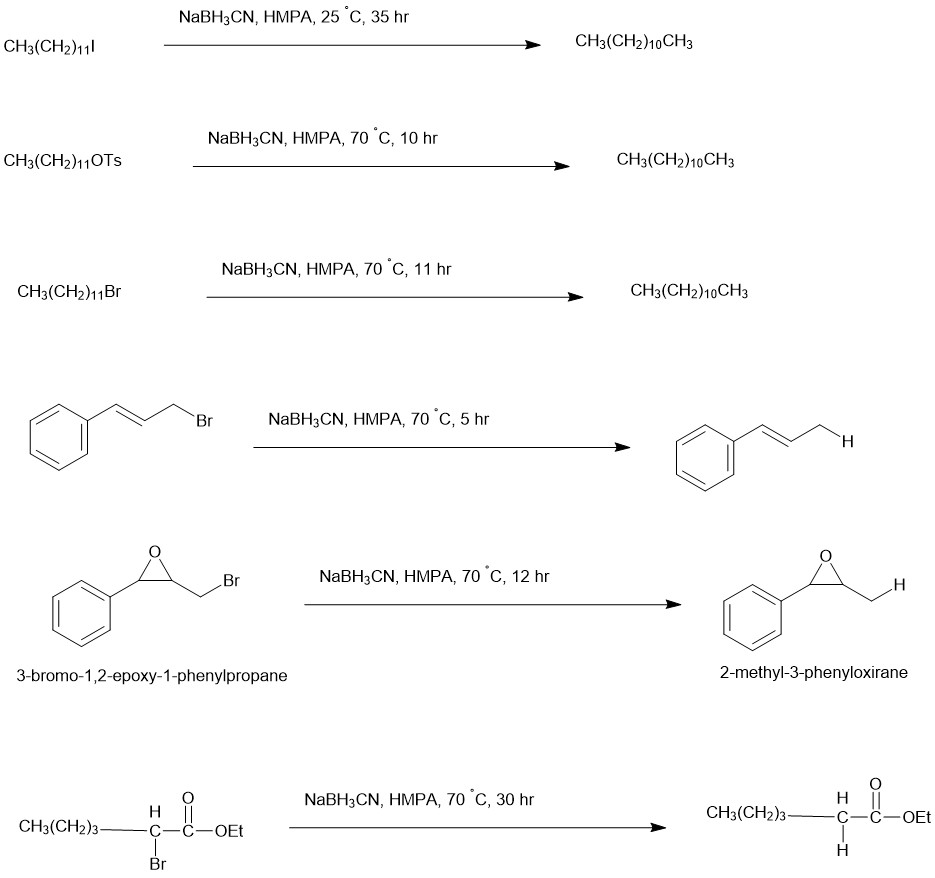
In yet another method, Hutchins and coworkers reported the deoxygenation of alcohols via the reduction of iodides, bromides or tosylates using sodium cyanoborohydride in Hexamethylphosphoramide (HMPA). The results were best accommodated by an SN2 process involving direct attack by hydride or cyanoborohydride anion.
In a typical experiment, the substrate (1 mmol) was dissolved in HMPA (5 mL) followed by the addition of NaBH3CN and stirred at temperatures of 25 °C to 100 °C.
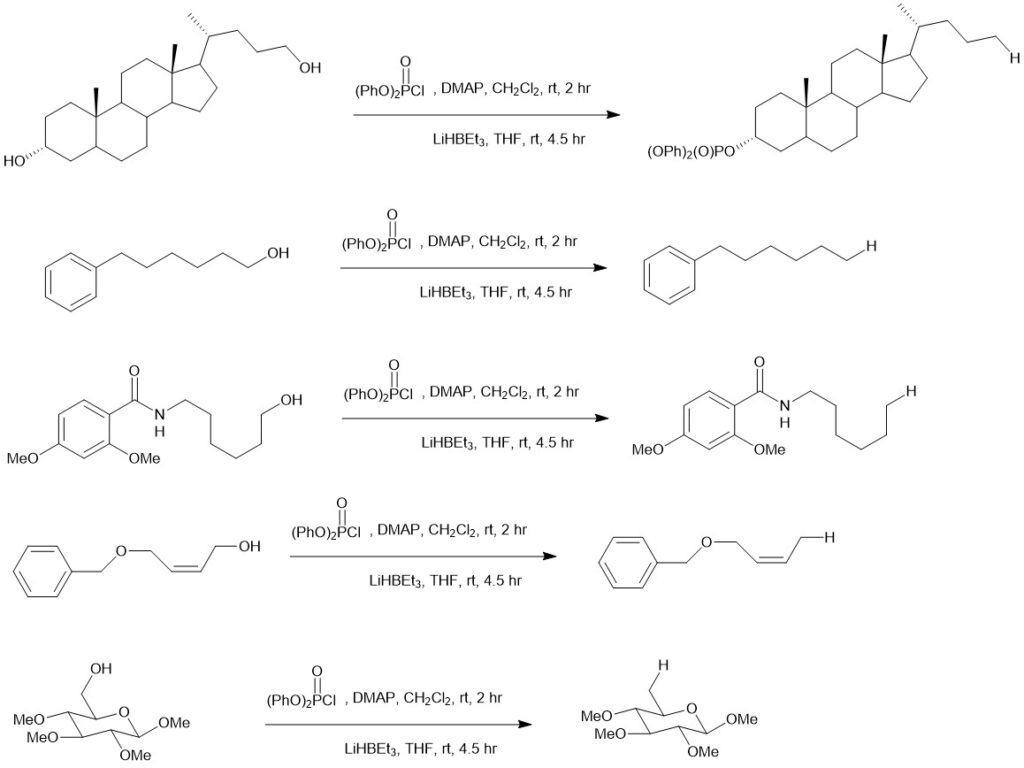
The reduction of primary alcohol-derived diphenyl phosphate esters with lithium triethylborohydride is also a method for deoxygenation of primary alcohols. In 2016, Standaert and co-workers reported deoxygenation of unhindered alcohols via reductive dealkylation of derived phosphate esters. The use of diphenyl phosphate esters allows for selective reduction of primary alcohols in the presence of secondary alcohols. Lithium triethylborohydride is a mild and selective reducing agent that can reduce various functional groups, including alcohols, ketones, aldehydes, and esters. It is also a popular reagent for deoxygenation. Performing the two-step process in one pot simplifies the reaction and makes it more convenient for synthetic chemists. Tetrahydrofuran is a common solvent used for the reaction due to its ability to solubilize both the reagents and the reaction products.
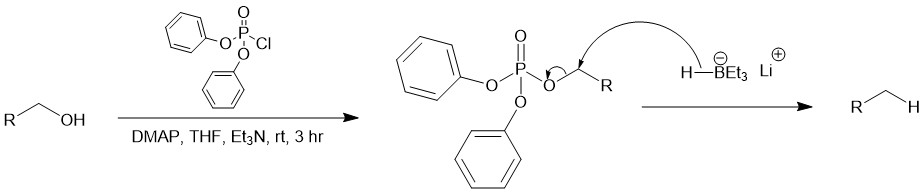
In a typical experiment, the first step involves the reaction of the alcohol with diphenyl chlorophosphate and N,N-dimethylaminopyridine (DMAP) in dichloromethane to generate the phosphotriester. Diphenyl chlorophosphate is a reagent commonly used for phosphorylation reactions, while DMAP (4-(dimethylamino)pyridine) is a catalyst often used in esterification and phosphorylation reactions. Dichloromethane is a common solvent for organic reactions. The second step involves the reduction of the phosphotriester using 1.7 equivalents of lithium triethylborohydride in THF. As mentioned earlier, lithium triethylborohydride is a mild and selective reducing agent that is commonly used for deoxygenation reactions.
- Chowdhury, S.; Standaert, R. F. Deoxygenation of unhindered alcohols via reductive dealkylation of derived phosphate esters. J. Org. Chem. 2016, 81, 9957-9963
- Hutchins, R. O.; Maryanoff, B. E.; Milewski, C. A. Sodium cyanoborohydride in hexamethylphosphoramide. An exceptionally selective reagent system for the reduction of alkyl iodides, bromides, and tosylates. J. Chem. Soc. Chem. Commun. 1971, 18, 1097-1098. 87
- Krishnamurthy, S.; Brown, H. C. Facile reduction of alkyl tosylates with lithium triethylborohydride. An advantageous procedure for deoxygenation of cyclic and acyclic alcohols. J. Org. Chem. 1976, 41, 3064-3066.



