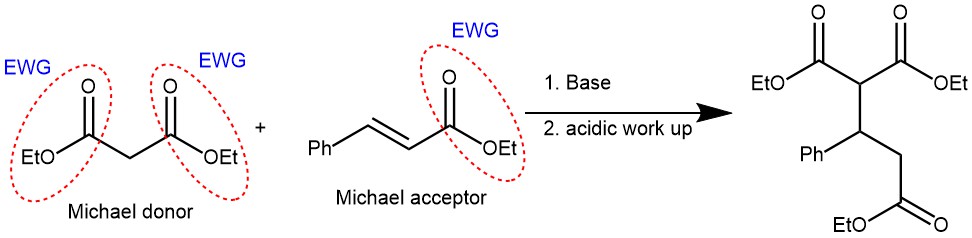
The Michael reaction is a versatile organic chemical reaction named after its discoverer, Arthur Michael. The nucleophilic addition of enolate anion (or analogous nucleophile) to the carbon–carbon double bond of α, β-unsaturated ketones, aldehydes, nitriles, or carboxylic acid derivatives is known as the Michael reaction or Michael addition reaction. Currently, all reactions that involve the 1,4-addition (conjugate addition) of virtually any nucleophile to activated π-system are also referred to as the Michael addition reaction.
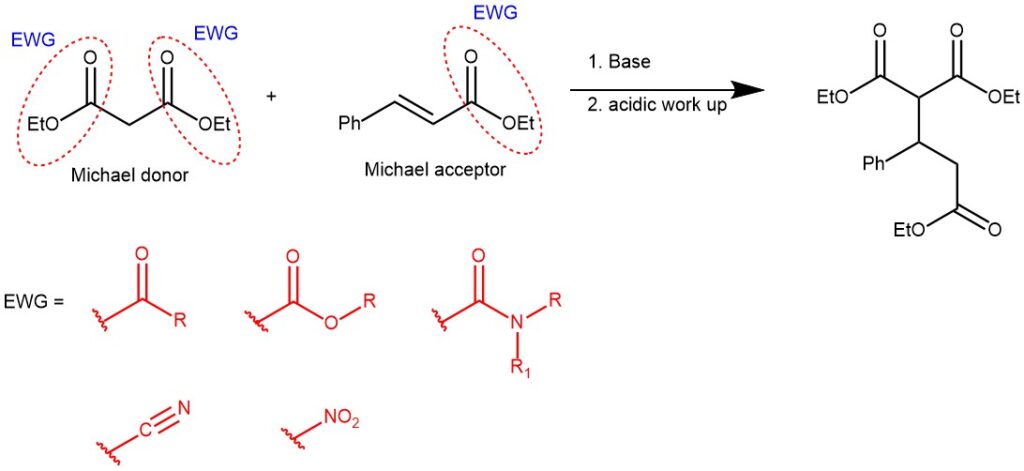
The α, β-unsaturated compound in the Michael Reaction is called Michael acceptor and could include any unsaturated system having a functional group capable of stabilizing the carbanionic intermediate.
On the other hand, Michael donors are the nucleophiles which can be derived by the deprotonation of CH-activated compounds such as aldehydes, ketones, β-dicarbonyl compounds, etc.
The base used are relatively weak bases like piperidine, pyridine, triethylamine, KOH, triton B. Sometimes stronger bases like sodium ethoxide, potassium tertiary butoxide, sodium hydride, sodium amide, etc. are also used.
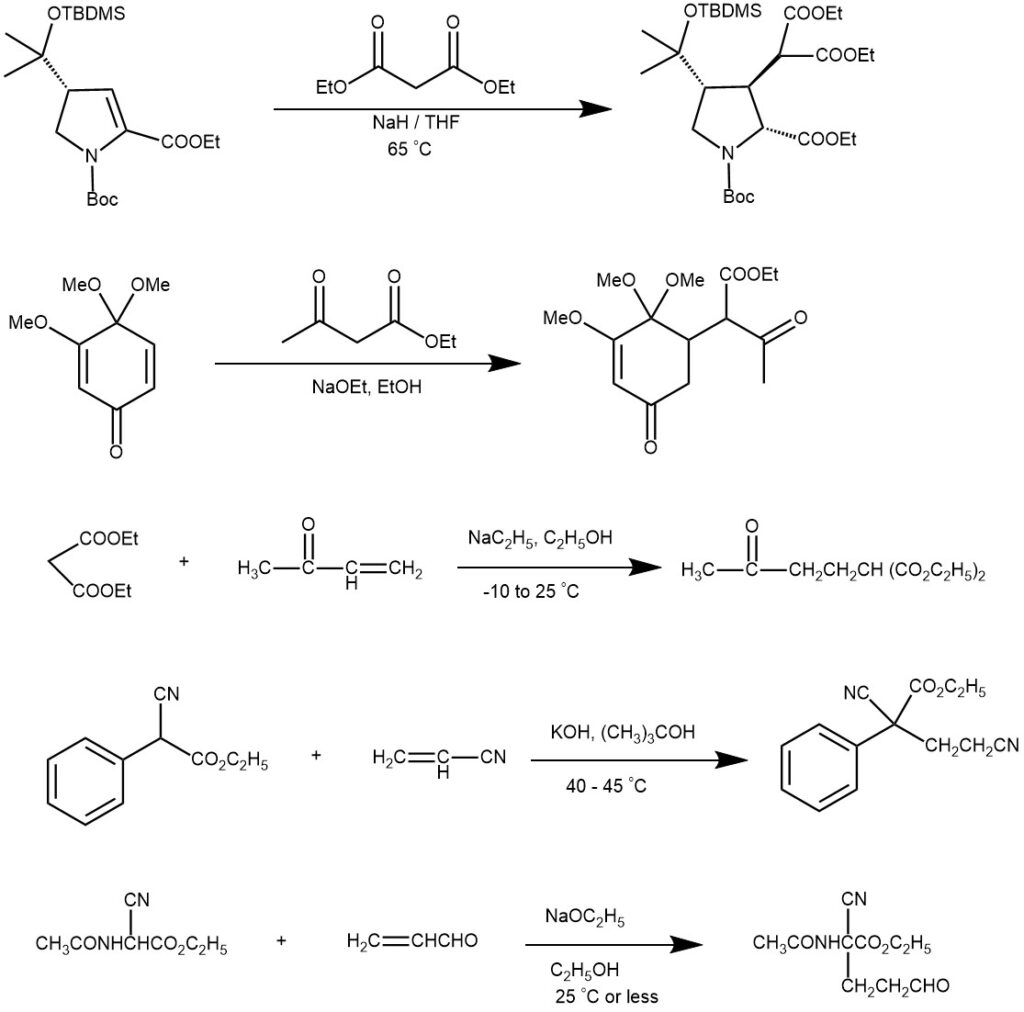
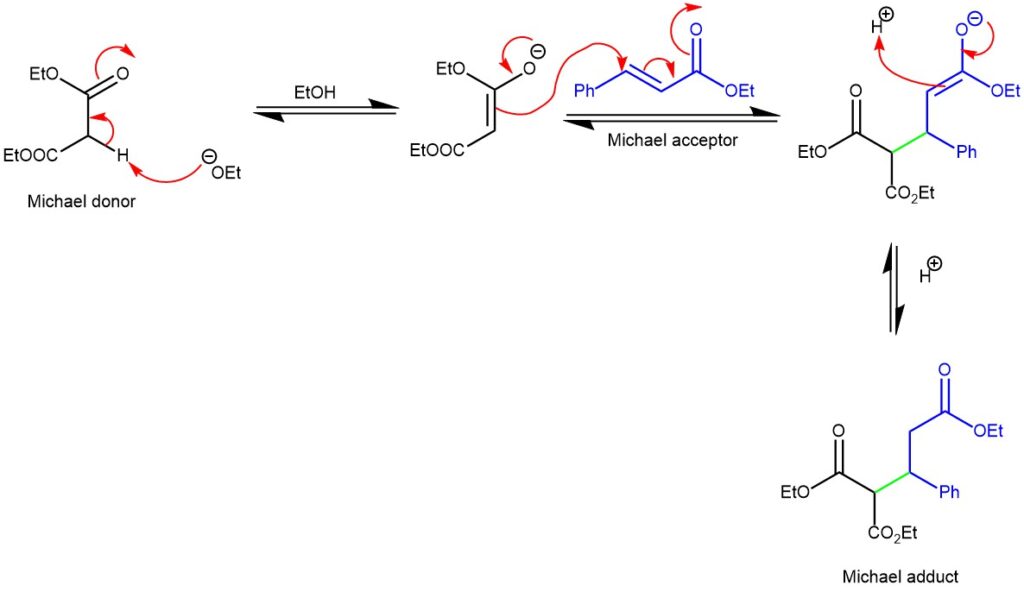
MECHANISM: The mechanism of the Michael addition involves the nucleophilic addition of a nucleophile (Michael donor) to an α, β-unsaturated carbonyl compound (Michael acceptor). A strong base, such as an alkoxide ion (RO⁻) or an amine, deprotonates from the α-carbon of the Michael donor. This generates an enolate intermediate, which is a resonance-stabilized nucleophile. The enolate nucleophile attacks the β-carbon of the Michael acceptor, typically an α, β-unsaturated carbonyl compound. The β-carbon is electrophilic due to the presence of the electron-withdrawing carbonyl group. The nucleophilic attack results in the formation of a new carbon-carbon bond.
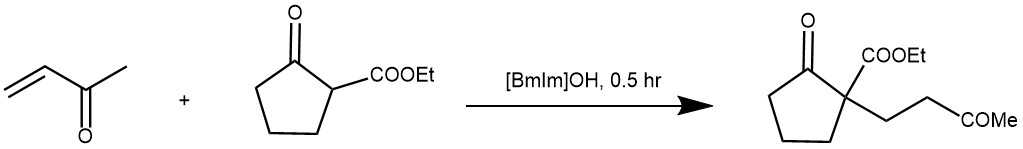
EXAMPLE 1: Methyl vinyl ketone (420 mg, 6 mmol) was added dropwise to a well stirred mixture of ethyl 2-oxo-cyclopentane carboxylate (755 mg, 5 mmol) in [bmIm]OH (468 mg, 3 mmol), and the reaction mixture was stirred for 0.5 h until completion of the reaction (TLC). The product was then distilled off directly from the reaction mixture to provide pure Michael adduct (1.08 g, 96%) as a colorless oil. If the reaction was carried out on a relatively smaller scale (1-2 mmol), the reaction mixture was extracted with ethyl acetate. The extract was washed with brine, dried (Na2SO4), and evaporated, leaving the crude product, which was purified by column chromatography over silica gel to provide the pure product. [REF: Org. Lett., Vol. 7, No. 14, 2005]
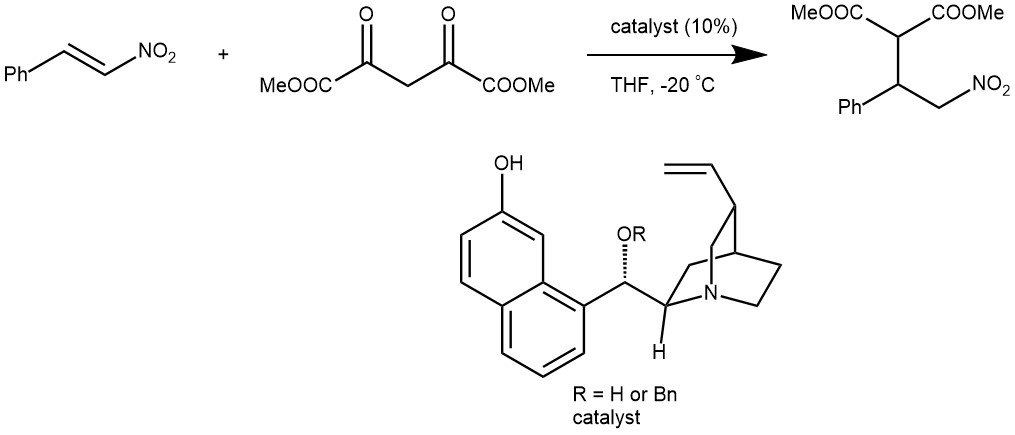
EXAMPLE 2: At -20°C, to a solution of a nitroalkene (0.2 mmol) in THF (0.2 mL) was added dimethyl malonate (68 μL, 3 eq.) and the chiral catalyst (6.2 mg, 10 mol%.). The resulting mixture was kept at -20 °C until nitroalkene is consumed. The reaction mixture was then passed through a plug of silica gel for the removal of the catalyst (eluent: ether or ethyl acetate). The filtrate was concentrated in vacuo and the residue was purified by flash chromatography. [REF: J. AM. CHEM. SOC. 2004, 126, 9906-9907]
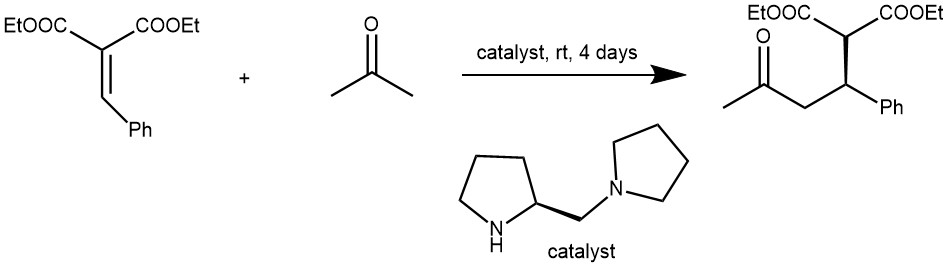
EXAMPLE 3: To a solution of diethyl benzalmalonate (62 mg, 0.25 mmol) in a mixture of THF (2 mL) and acetone (0.5 mL) was added (S)-1-(2-pyrrolidinylmethyl)-pyrrolidine (8.5 L, 20% mol). The reaction was stirred at room temperature for 4 days. Then, the solution was diluted with CH2Cl2 (5 mL) and treated with 1N HCl (4 mL) with vigorous stirring. The layers were separated, and the aqueous phase was extracted thoroughly with CH2Cl2 (3×2 mL). The combined organic phases were dried (MgSO4), concentrated and purified by flash column chromatography on silica gel affording the Michael adduct in 47% yield (36 mg, 0.12 mmol). [REF: Tetrahedron Letters 42 (2001) 4441–4444]
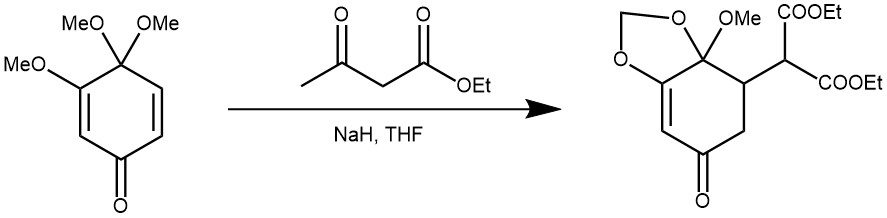
EXAMPLE 4: To a stirred suspension of NaH (34 mg, 50% oil dispersion, 0.71 mmol, washed with n-hexane three times) in 1 mL of dry THF was added cyclohexadienone (112mg, 0.67 mmol) in 1 mL of dry THF and diethyl malonate (110 mg, 0.68 mmol) in 1 mL of dry THF. The reaction mixture was stirred at 35 °C for 17 h and quenched with wet ether. Solvent was evaporated and the residue was partitioned between ether and H20. The organic phase was washed with H20 and saturated NaCl solution and dried over MgSO4. Concentration and preparative TLC on silica gel with benzene/ether (8:1) as eluant gave96 mg (44%) of an oil. [REF: J. Org. Chem., Vol. 45, No. 7, 1980]
REFERENCES:
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako
- Modern synthetic reactions by Herbert O. House