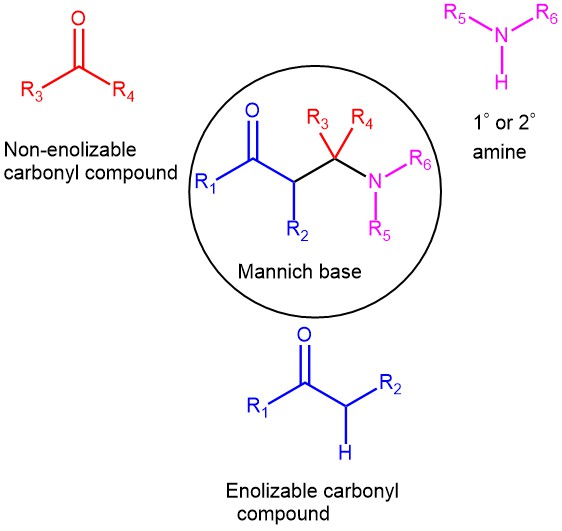
The condensation of a CH-activated compound (usually an aldehyde or ketone) with a primary or secondary amine (or ammonia) and a non-enolizable aldehyde (or ketone) to afford β-amino ketone is known as the Mannich reaction. In this transformation, three components, a ketone, an aldehyde, and an amine, react to form a β-amino ketone. The product β-amino ketone is also known as the Mannich Base. Only primary and secondary aliphatic amines or their salts are used. Aromatic amines tend not to react. The non-enolizable component in most cases is formaldehyde while the CH-active component is usually an aliphatic aldehyde or ketone, carboxylic acid derivatives, β-dicarbonyl compounds, nitroalkanes, etc. The solvents are usually water, methanol, ethanol, and acetic acid.
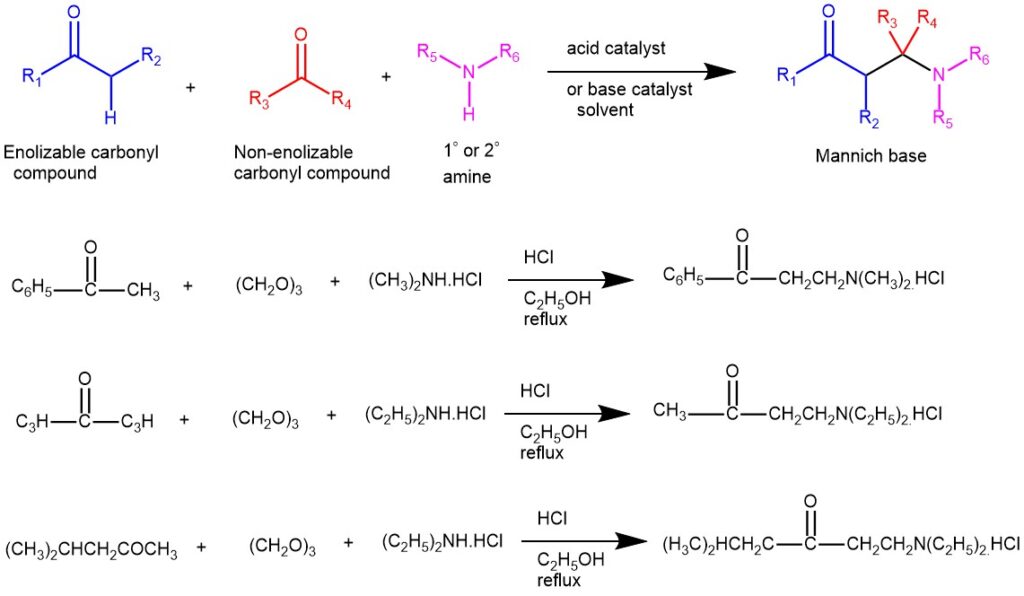
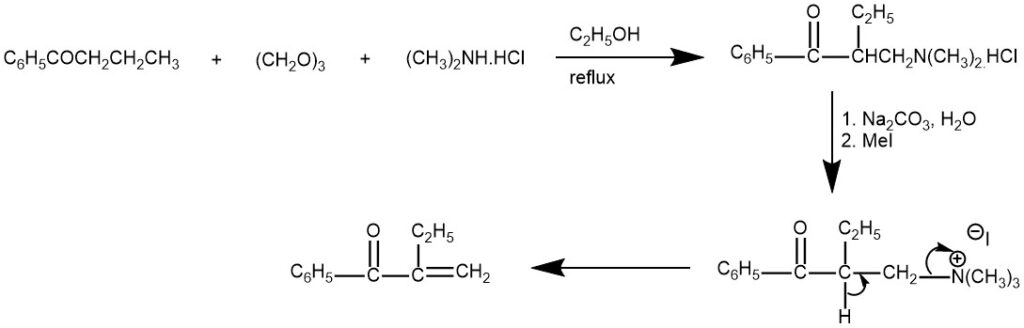
Mannich bases are useful synthetic intermediates. β-elimination from the Mannich base forms α, β-unsaturated ketones, and also Michael acceptor.
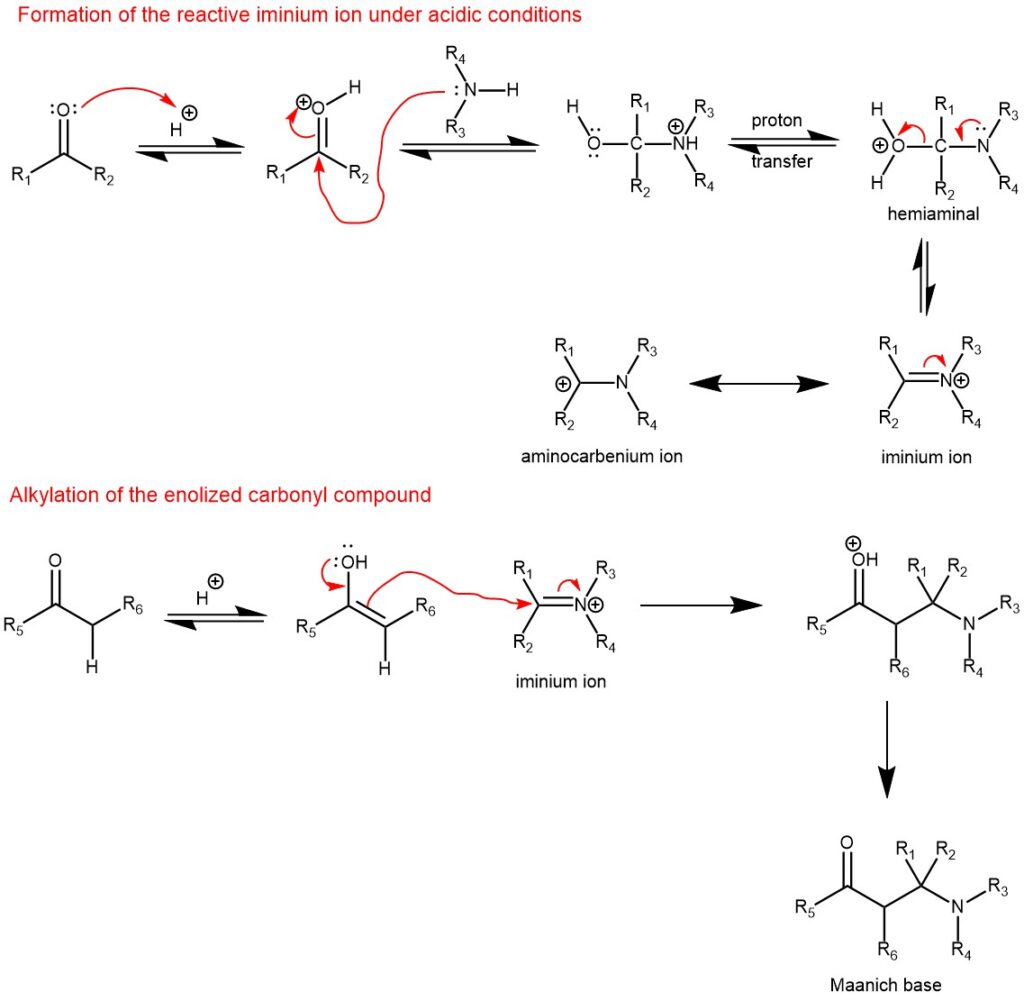
MECHANISM: Under slightly acidic reaction conditions, the first step is the reaction of the amine with the protonated non-enolizable carbonyl compound to form electrophilic iminium salt. The electrophile attack by the iminium salt on the enol of the active methylene compound (enolizable carbonyl compound) gives rise to the Mannich base.
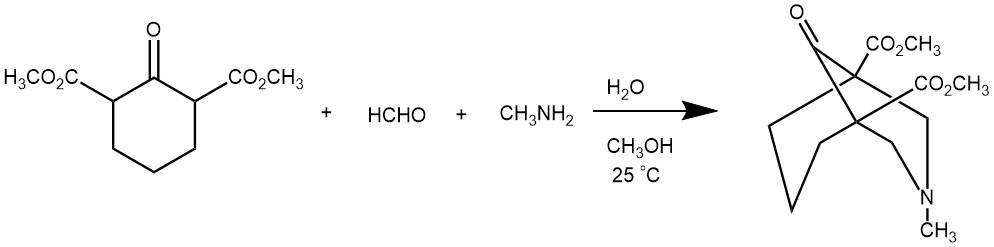
EXAMPLE 1: A solution of 6.0 g. (0.028 mol.) of dimethyl cyclohexanone-2,6-dicarboxylate in 200 ml. of methanol was stirred and 3.5 g. of 25% aqueous methylamine (0.028 mole) and 4.5 g. of 37% formalin (0.056 mole) were added. The mixture was stirred for 24 hr. and the methanol and water were then removed under reduced pressure; yield 6.0 g. (80%); m.p. 76-78°. After two more recrystallizations from methanol, the product melted at 80-81°.[REF: J. Org. Chem. 1959, 24, 9, 1379–1380]
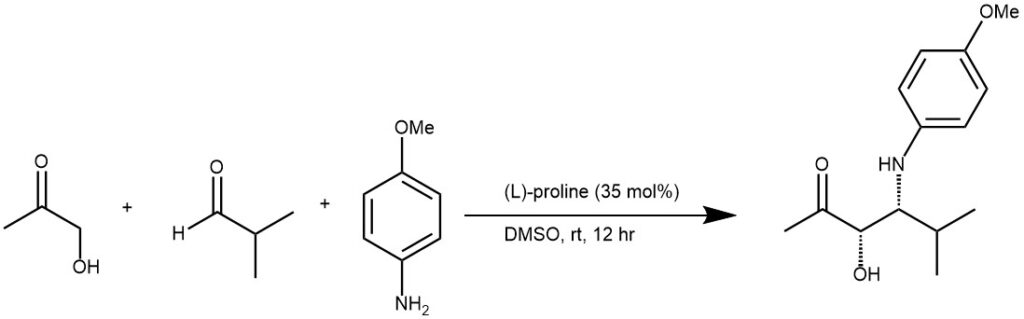
EXAMPLE 2: A suspension of L-proline (800 mg, 7 mmol) in 200 mL of DMSO/acetone (4:1) was treated with p-anisidine (2.7 g, 22 mmol) and isobutyraldehyde (1.44 g, 20 mmol) at room temperature and stirred for 12 hours. The mixture was poured into phosphate buffer (pH 7) and ethyl acetate, and the aqueous layer was extracted three times with ethyl acetate. The organic layers were dried over anhydrous MgSO4, filtered, and concentrated to furnish after column chromatography (hexanes/ethyl acetate 6:1- 3:1) syn-A (2.7 g, 10.8 mmol, 54%) and anti-A (151 mg, 0.6 mmol, 3%) as oils.[REF: J. Am. Chem. Soc. 2000, 122, 9336-9337]
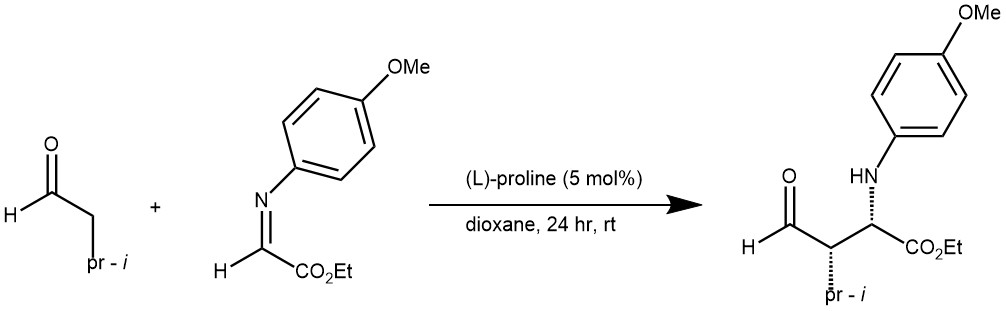
EXAMPLE 3: In a typical experiment, N-PMP-protected α-imino ethyl glyoxylate (0.5 mmol) was dissolved in anhydrous dioxane and the corresponding aldehyde donor (0.75 mmol) was added, followed by L-proline (5 mol%). The total volume of the reaction mixture was 5 mL. After stirring for 2-24h at room temperature, the mixture was worked up by the addition of a half-saturated ammonium chloride solution and extraction with ethyl acetate. The combined organic layers were dried (MgSO4), filtered, concentrated, and the residue purified by column chromatography (silica, hexanes/ethyl acetate mixtures) to afford the corresponding Mannich addition product. The ee’s of all products were determined by chiral HPLC analysis.[REF: VOL. 124, NO. 9, 2002 9 J. AM. CHEM. SOC.]
REFERENCES:
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako
- Modern synthetic reactions by Herbert O. House