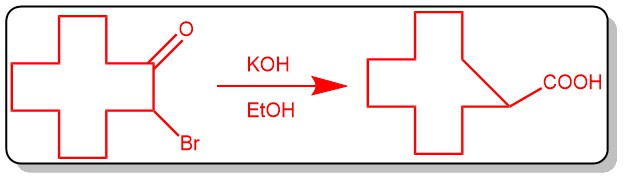
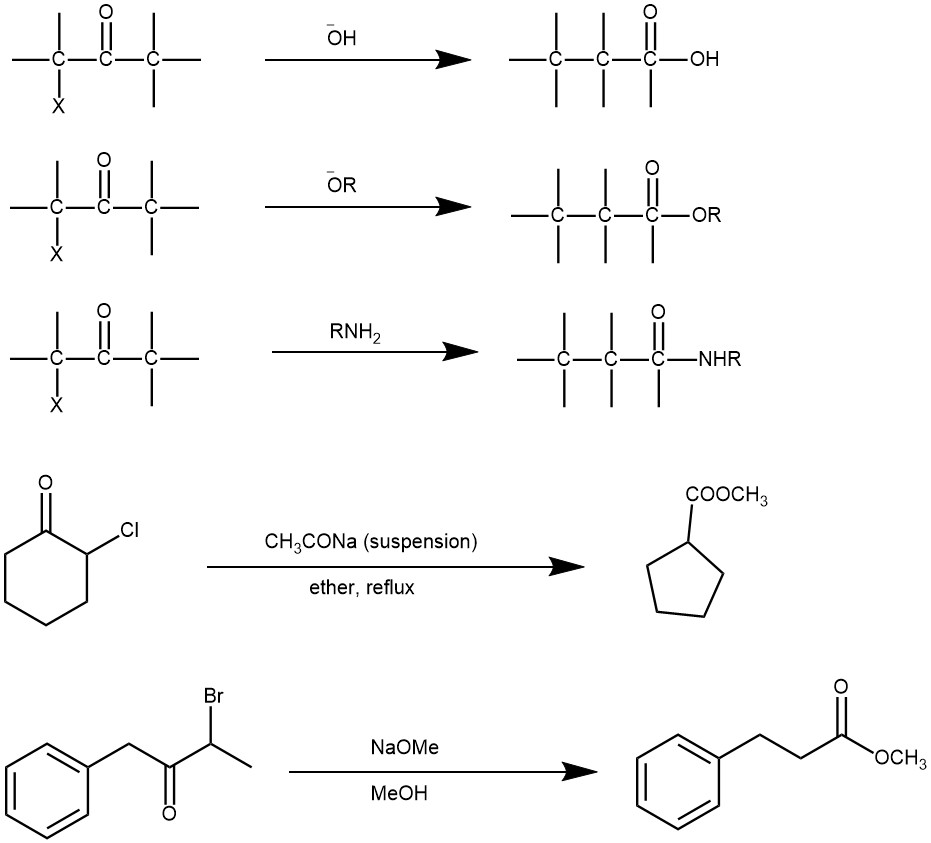
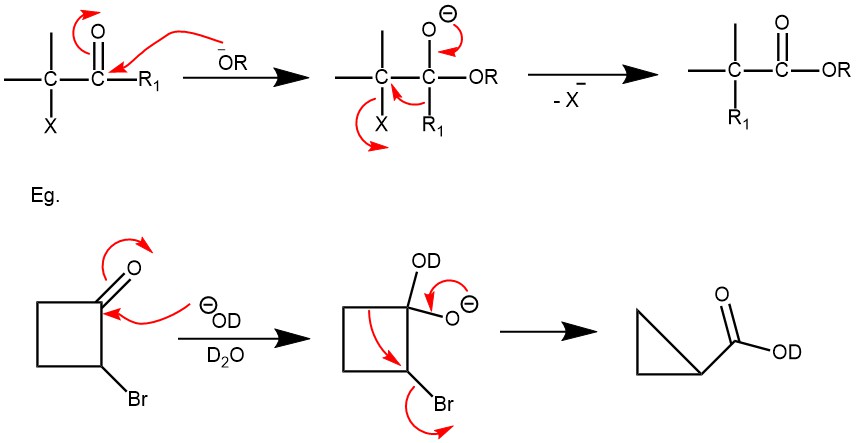
It is a base-catalyzed rearrangement of α-halo ketones with at least one α-hydrogen to form a carboxylic acid, an ester, or an amide is called the Favorskii rearrangement. The product formed depends upon the base used: hydroxide (-OH‾) gives carboxylic acid (-COOH), alkoxide(RO‾) gives an ester (-COOR), and the use of an amine(-NH2) gives an amide (-CONH2). Upon rearrangement, acyclic α-halo ketones give acyclic carboxylic acid derivatives, while cyclic α-halo ketones react to afford one cabon smaller cyclic carboxylic acid derivatives.
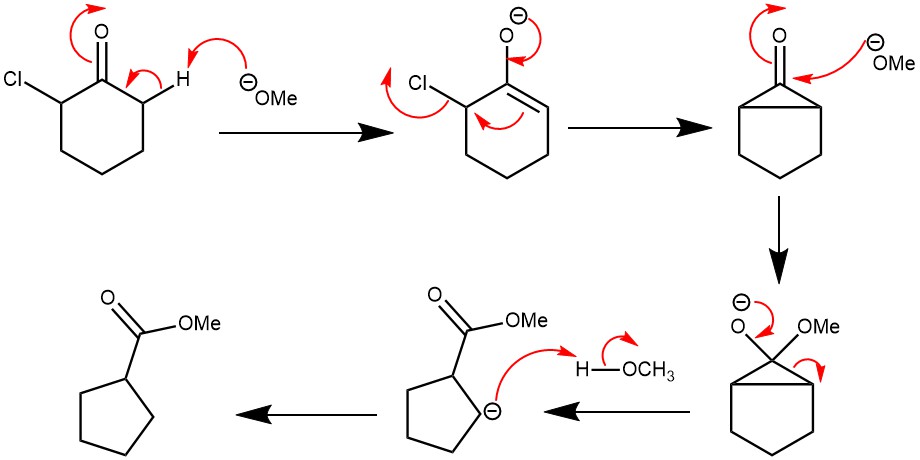
MECHANISM: The mechanism of the reaction is determined by the presence of an α-hydrogen. The base abstracts a proton from the α-carbon, generating an enolate intermediate. Intramolecular attack by the enolate on the α-carbon bearing the halogen forms a cyclopropenone intermediate. This is followed by the regioselective opening of the intermediate by a nucleophile to give the most stable carbanion and proton transfer afford the product.
When the halo ketones have no α-hydrogen the reaction is called the abnormal or Quasi-Favorskii rearrangement. The mechanism operating in these substrates is called the Semibenzilic mechanism. It was first proposed by Tschoubar and Sackur in 1939. This mechanism involves the addition of alkoxide to the carbonyl carbon of the halo ketone, followed by a concerted displacement of halide ion by the 1,2-migration of an alkyl group with its electron pair.
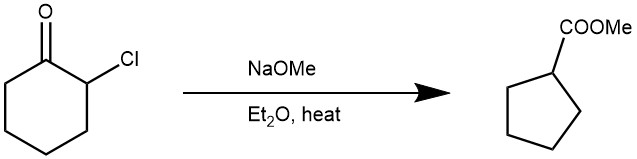
EXAMPLE 1: A dry 1L. three-necked, round-bottomed flask is equipped with an efficient stirrer, a spiral reflux condenser, and a dropping funnel, and all openings are protected by calcium chloride drying tubes. A suspension of 58 g. (1.07 moles) of sodium methoxide in 330 ml. of anhydrous ether is added, and stirring is begun. To the stirred suspension is added dropwise a solution of 133 g. (1 mole) of 2-chlorocyclohexanone diluted with 30 ml. of dry ether. The exothermic reaction is regulated by the rate of addition of the chloroketone; about 40 minutes is required for the addition. After the addition of the chloroketone is complete, the mixture is stirred and heated under reflux for 2 hours and is then cooled. Water is added until the salts are dissolved. The ether layer is separated, and the aqueous layer is saturated with sodium chloride. After extraction of the aqueous layer two 50-ml. portions of ether, the ethereal solutions are combined and washed successively with 100-ml. portions of 5% hydrochloric acid, 5% aqueous sodium bicarbonate solution, and saturated sodium chloride solution. The ether solution is dried over magnesium sulfate, and the magnesium sulfate is removed by filtration and washed with ether. Removal of the ether by distillation at atmospheric pressure leaves the crude ester, which is distilled, with fractionation, at 70–73°/48 mm. The yield of methyl cyclopentanecarboxylate is 72–78 g. (56–61%).[REF: Organic Syntheses, Coll. Vol. 4, p.594 (1963); Vol. 39, p.37 (1959)]
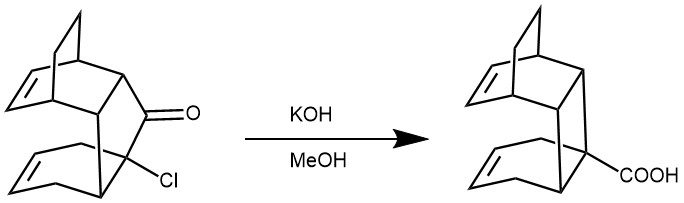
EXAMPLE 2: To a MeOH solution (10 mL) of the ketone (226 mg; 0.91 mmol) was added KOH (2.5 g) in H2O (5 mL) and the mixture was refluxed for 12 h. The mixture was acidified with dilute HC1 and extracted with ether. After being dried over MgSO4, the organic layer was heated to evaporate a volatile material, and the residue thus obtained was chromatographed on a silica-gel column to give the product (189 mg, 88%): colorless prisms; mp 162-163 °C.[REF: J. Org. Chem. 1994, 59, 6490—6492]
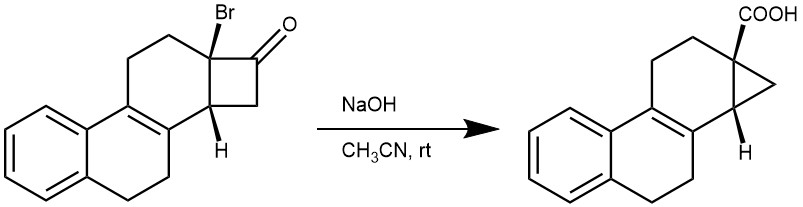
EXAMPLE 3: The bromo ketone (1 equiv, 33 mg, 0.1 mmol) was dissolved in CH3CN (2 mL) at ambient temperature. Aqueous NaOH (1.0 mL, 1.0 M) was added dropwise. The clear, colorless solution quickly developed an intense yellow color upon the addition of the base and became red over several minutes. After 5 min, the reaction was quenched by the addition of aqueous HCl (1 mL, 1.0 M). The aqueous mixture was extracted with Et2O, and the combined organics were washed with water and brine, dried (MgSO4 ), filtered, and concentrated in vacuo. The crude residue was purified by flash column chromatography (1:1, pentane/ether) to provide the product (22 mg, 80%) as a colorless oil: Rf = 0.54 (1:1 pentane/diethyl ether)[REF: dx.doi.org/10.1021/jo302230m | J. Org. Chem. 2013, 78, 204−210]
REFERENCES:
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako
- Modern synthetic reaction by Herbert O. House
- J. Chem. Educ. 1978, 55, 5, 286