Esterification is widely regarded as an essential organic transformation as these are critical for the synthesis of many commercially available drugs and building blocks. Carboxylic esters are a class of organic compounds containing -COO- functional group. Esters are generally synthesized by one of the four fundamental routes.
- Esterification of a carboxylic acid with an alcohol
- Alcoholysis of acid chlorides or anhydrides
- Transesterification
- The reaction of carboxylate salt with an alkyl halide or sulfate
Fischer Esterification:
Conversion of a carboxylic acid and alcohol to an ester in the presence of an acid catalyst, most commonly, sulfuric acid or para-toluene sulfonic acid (TsOH), or gaseous hydrogen chloride (HCl) is called as Fischer Esterification.
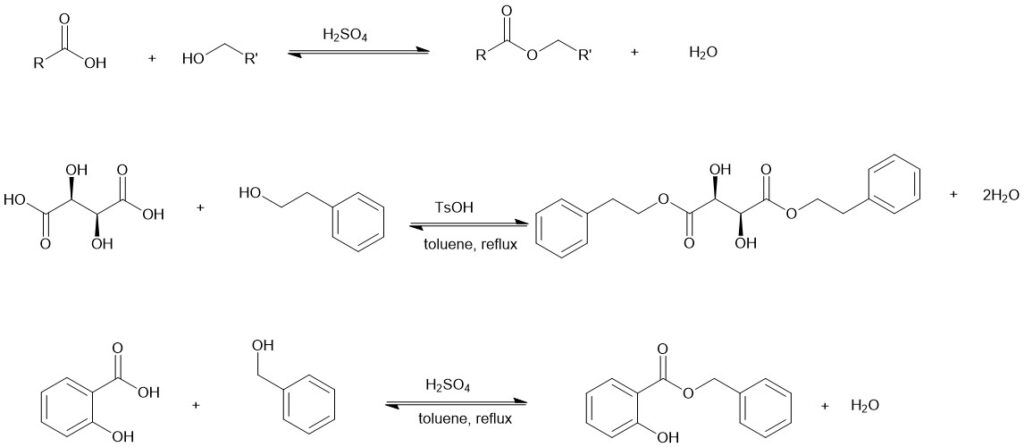
The Fischer esterification is reversible and can be accomplished if there is a means of driving the equilibrium in the forward direction. Most commonly, water is removed by azeotropic distillation and a Dean-Stark trap. In other instances, the addition of an excess of one of the reactants, usually alcohol, is employed to drive the equilibrium in the forward direction.
Mechanism: The Fischer esterification proceeds by nucleophilic attack of the alcohol on the protonated carbonyls of the carboxylic acids.
Alcoholysis of acyl halide and acid anhydride:
The reaction between acyl halide (acid halide) and alcohol is one of the best and quick methods for the preparation of carboxylic esters. This reaction, however, requires a base to combine with the acid formed in the reaction. Triethyl amine or pyridine works well.
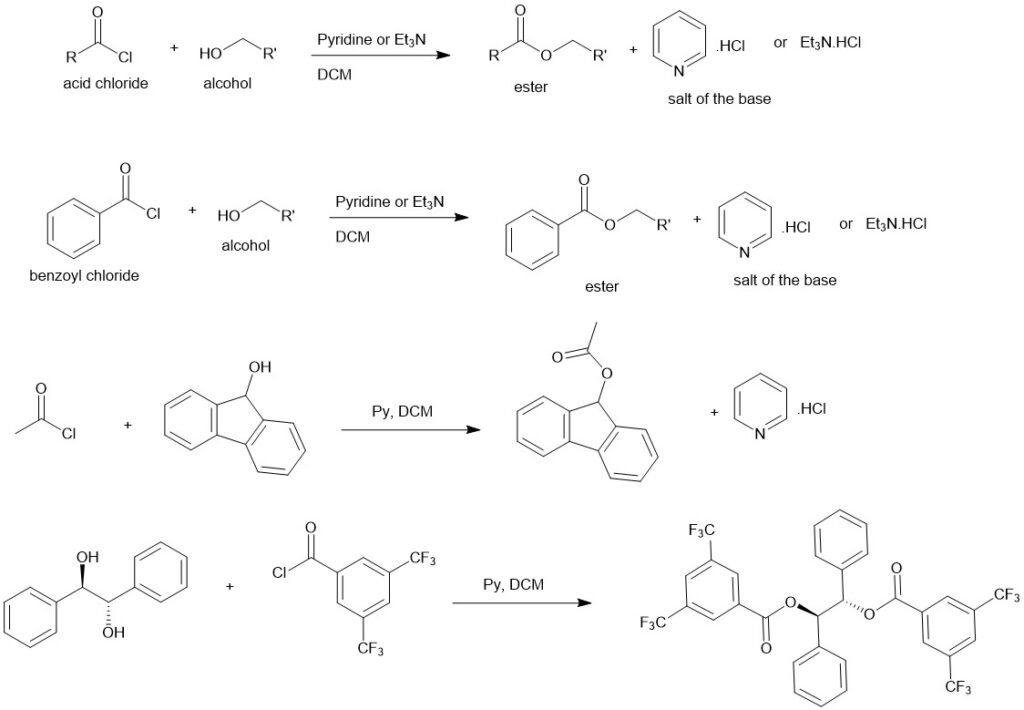
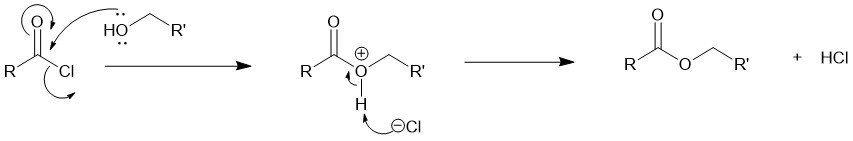
Mechanism: The addition-elimination mechanism is followed. The lone pair of electrons on the oxygen of the alcohol attacks the electrophilic carbonyl carbon of the acid chloride followed by the elimination of the chloride ion. The chloride ion acts as a base and pulls out the proton to form HCl and the final product.
Though acid anhydrides are less reactive than acyl halides, they are often used to prepare carboxylic esters. Pyridine is commonly used but 4-(N, N-dimethylamino)pyridine is a better catalyst where pyridine fails. It follows almost similar mechanistic pathways.
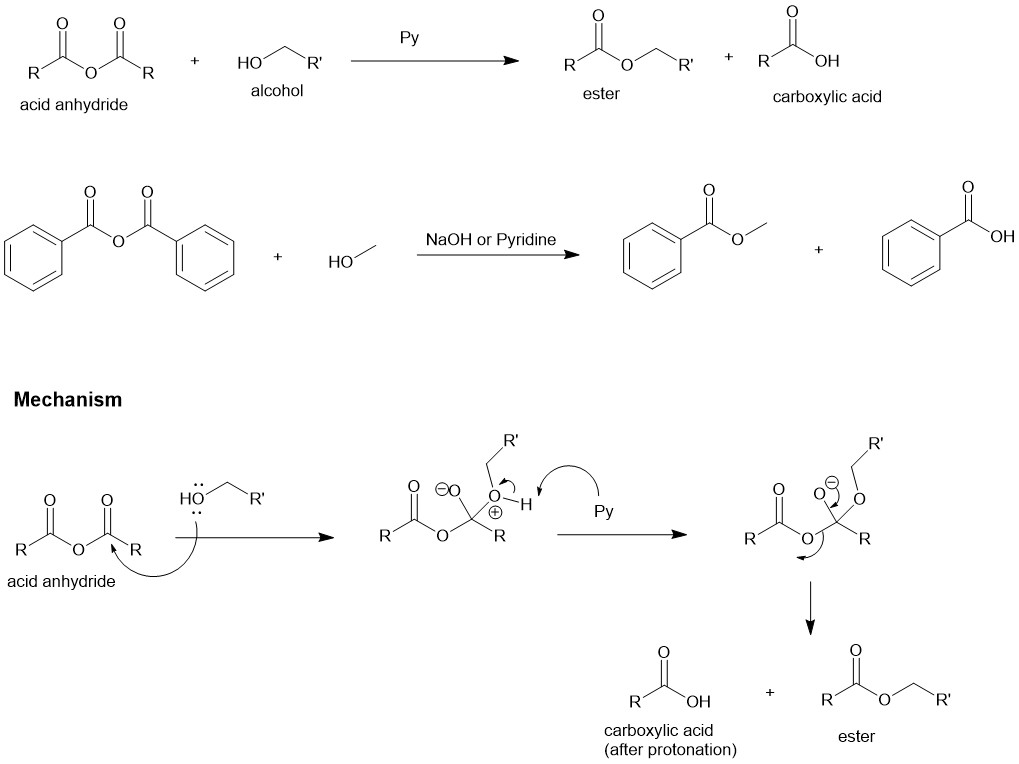
Mitsunobu Reaction:
The substitution of primary and secondary alcohols with nucleophiles in the presence of a dialkyl azodicarboxylate [eg, diethyl azodicarboxylate (DEAD)] and a trialkyl or triaryl phosphine [eg. Triphenylphosphine (PPh3)] is known as the Mitsunobu reaction. The primary and secondary alcohols are best suited for this reaction. When secondary alcohol is used there is an inversion of configuration ( as will be seen in the mechanism). The nucleophiles are relatively acidic compounds ( pKa £ 15) since the reagent (DEAD) must be protonated during the reaction to prevent the side-reactions. When a carboxylic acid is a nucleophile, esters are the product.
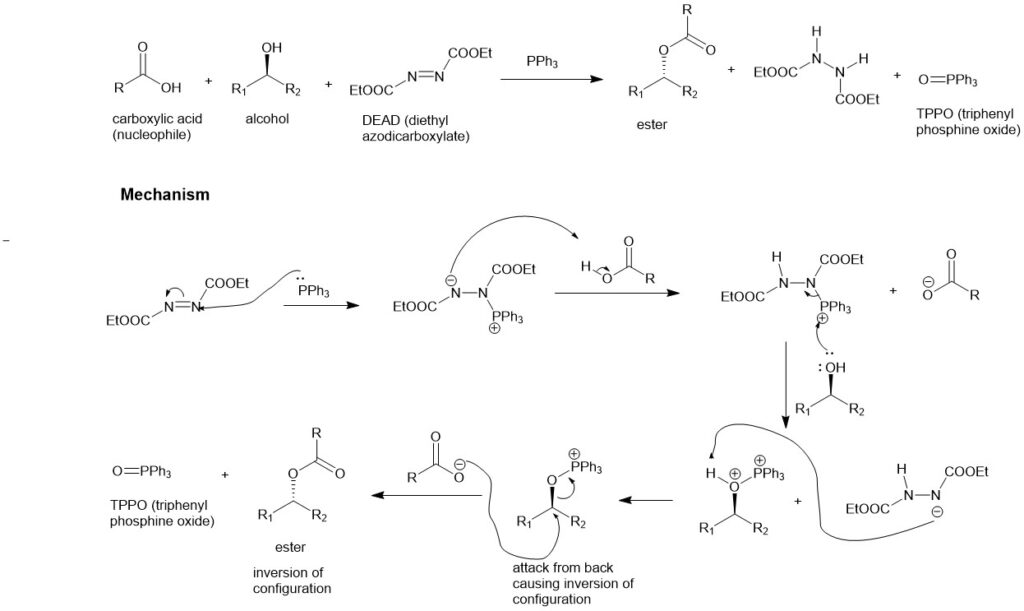
Yamaguchi esterification:
This reaction provides functionalized esters from carboxylic acids and alcohols employing 2, 4, 6-trichlorobenzoyl chloride as the condensation reagent in the presence of DMAP (4-N, N-dimethylaminopyridine). High yield, short reaction time, mild conditions, and highly functionalized esters are the noteworthy features of this reaction.
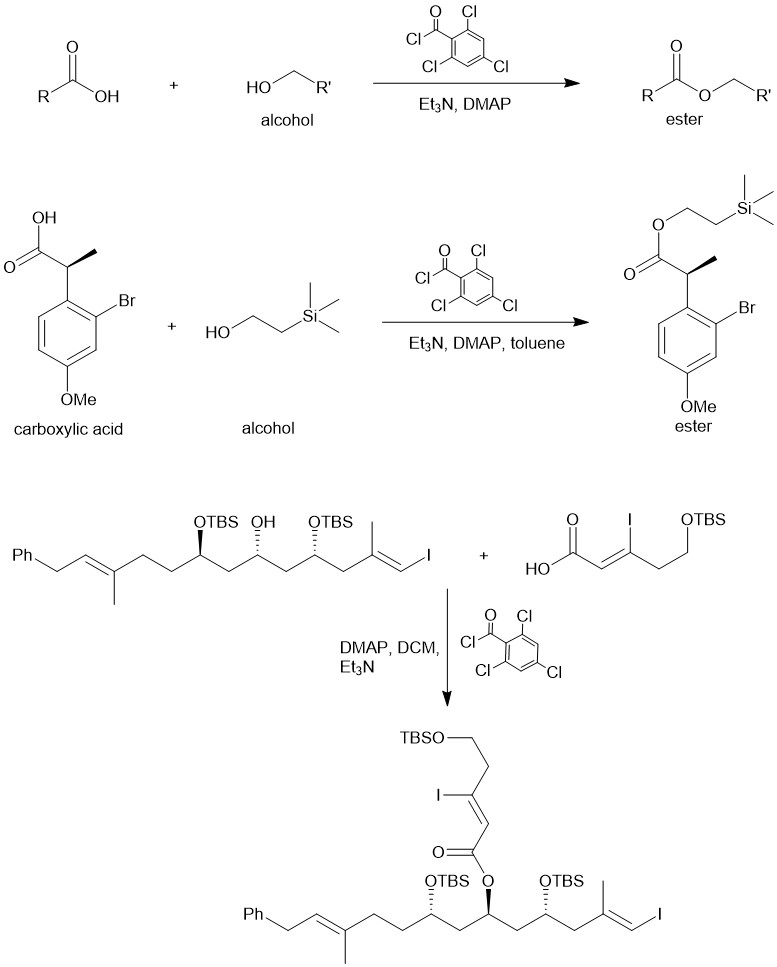
Mechanism:
- Formation of mixed anhydride between carboxylic acid and 2,4,6-trichlorobenzoyl chloride.
- DMAP as an acyl transfer reagent. DMAP is a stronger nucleophile than alcohol so it reacts with the mixed anhydride preferentially.
- Nucleophilic attack of the alcohol to the intermediate acylated DMAP. The acyl group is still polarized and the DMAP is a good leaving group.
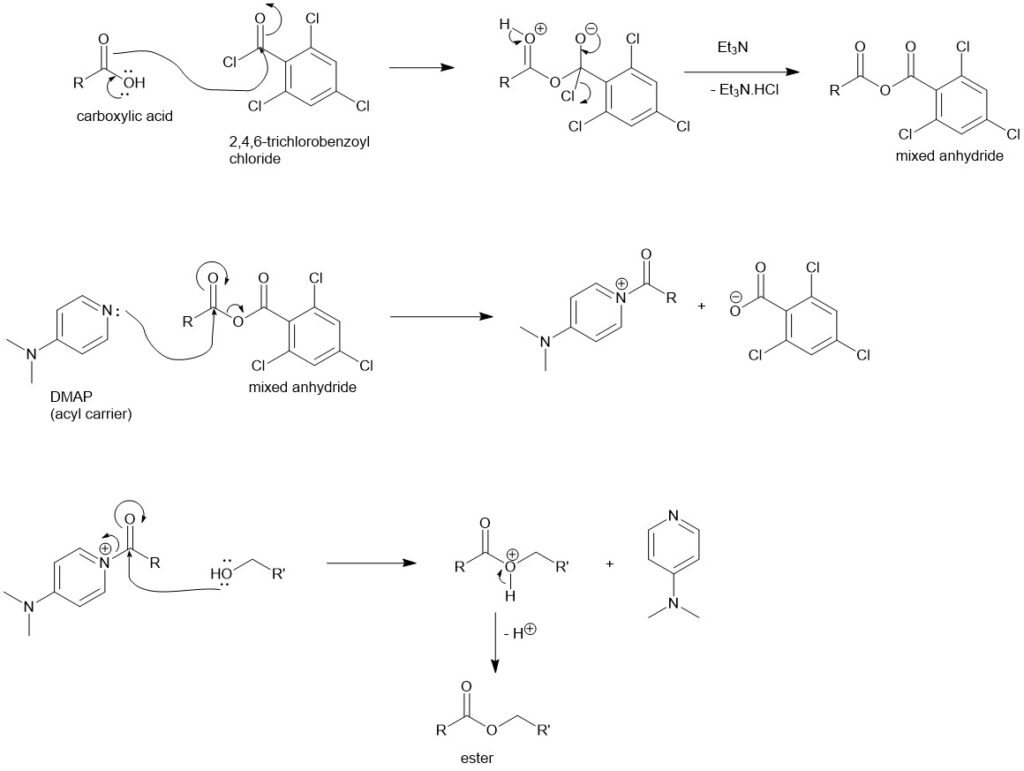
Steglich esterification:
In 1978, Steglich and Neises reported this method using DCC (N, N-dicyclohexylcarbodiimide) and DMAP (4-N, N-dimethylaminopyridine) with carboxylic acid and alcohol. This provides a wide range of esters containing acid labile, sterically hindered tert-butyl group, and so on. A catalytic amount of DMAP is generally used. DMAP accelerates the reaction rate by reacting with the O-acylisourea intermediate to form a highly activated electrophilic acylated pyridinium intermediate.
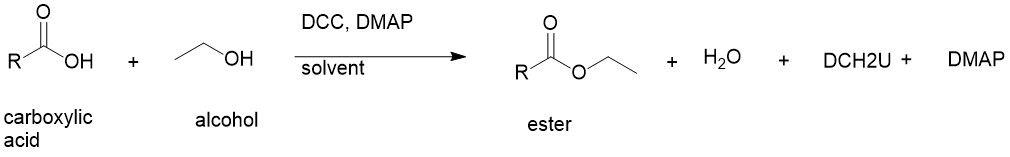
Mechanism:
- DCC and carboxylic acid react to form an O-acylisourea intermediate with reactivity higher than the carboxylic acid.
- DMAP reacts with the O-acylisourea to form a reactive amide intermediate (DMAP as an acyl carrier). The alcohol reacts with this amide to form the ester releasing very stable N,N’-dicyclohexyl urea (DCU) and the DMAP
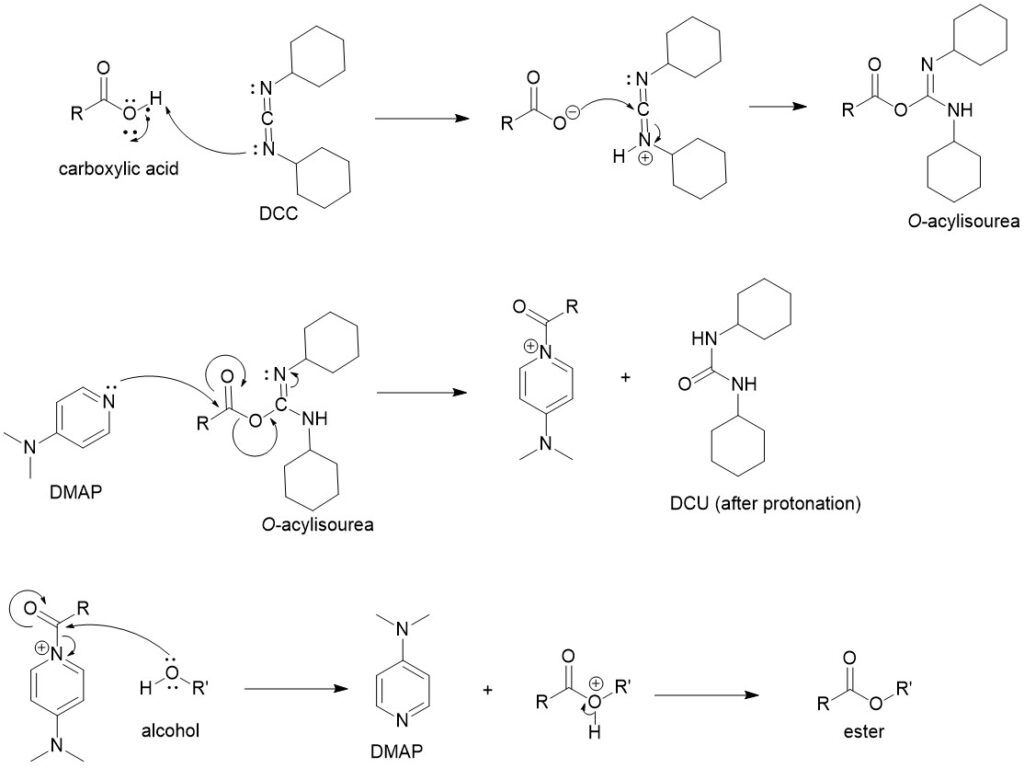
Diazomethane:
Carboxylic acids can be converted to esters with diazo compounds. The reactivity increases with the acidity of the carboxylic acids and when diazomethane is used, the carboxylic acids can be converted into corresponding methyl esters. This method is generally employed only when high yields are important or the acid is sensitive to higher temperatures.
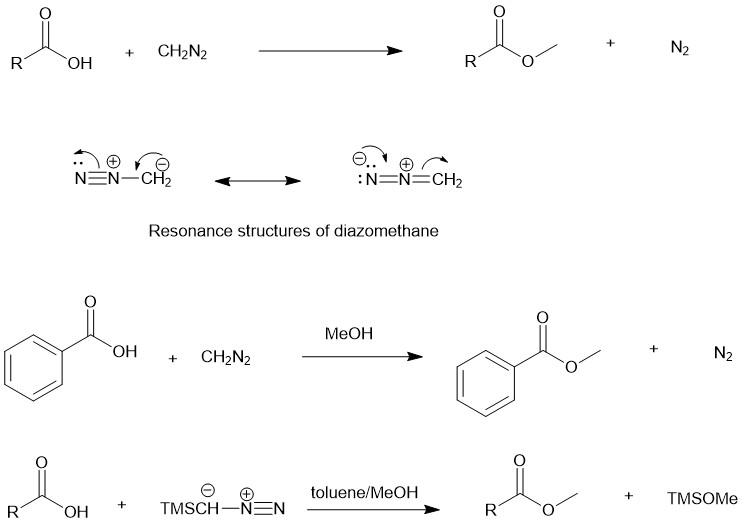
The diazomethane is first protonated by the carboxylic acid forming a carboxylate ion which then attacks the methyl group forming the ester and releasing nitrogen gas. In pure form at room temperature, it is an extremely sensitive explosive yellow gas. Since diazomethane is explosive and carcinogenic, trimethylsilyl diazomethane-hexane solution is used as an alternative.
There are many other named and unnamed reactions which lead to ester formation which will be presented in subsequent articles.



