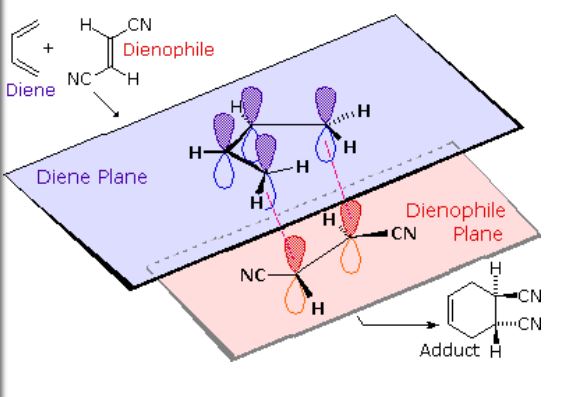
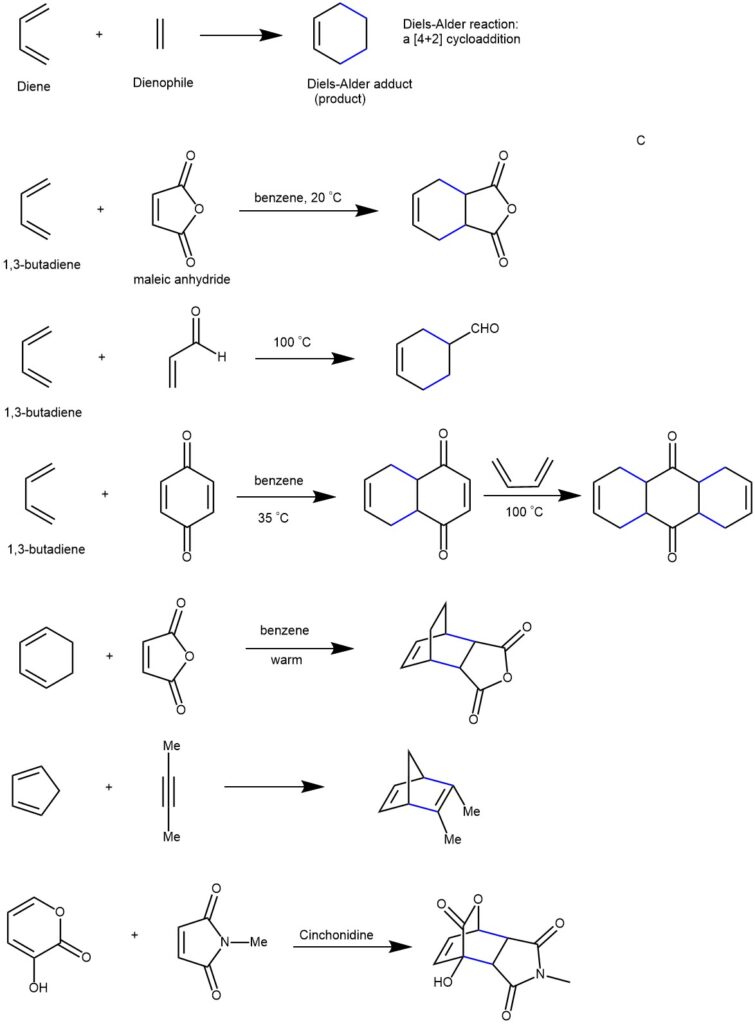
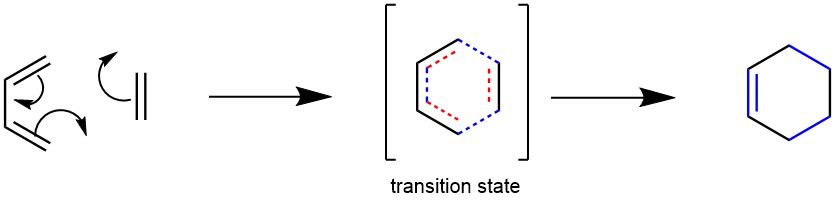
The Diels-Alder reaction is a chemical process between a conjugated diene and an alkene, also known as a dienophile. This reaction results in the formation of unsaturated six-membered rings (cyclohexene). Due to the creation of a cyclic product via a cyclic transition state, it is often referred to as a “cycloaddition” reaction. This pericyclic reaction involves the [4+2] cycloaddition of 4 π-electrons from the conjugated diene and 2 π-electrons from the dienophile (an alkene or alkyne). This reaction’s outcome involves forming new σ-bonds, which are more energetically stable than the initial π-bonds. Discovered in 1928 by German chemists Otto Diels and Kurt Alder, this reaction holds significant synthetic importance and earned them the Nobel Prize in 1950.
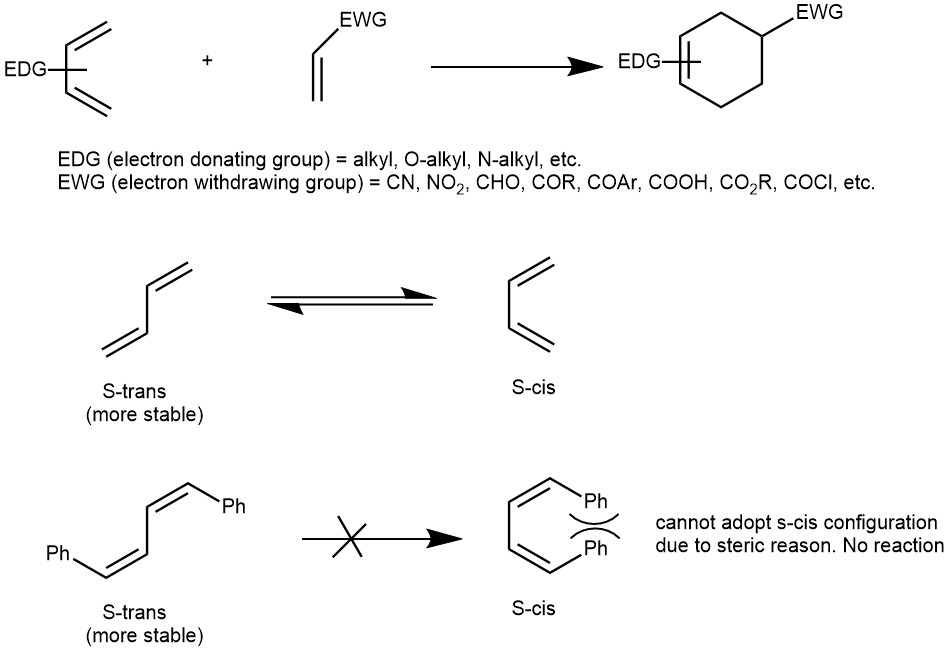
DIENE: In general, Diels-Alder reactions are most efficient when the diene component contains electron-donating groups (electron-rich), such as alkyl groups or alkoxy groups. The diene can exist in two conformations: S-cis and S-trans. The S-cis and S-trans forms are in rapid equilibrium. Though most of the acyclic dienes have a more stable S-trans conformation, they can still be utilized as reactants in Diels-Alder reactions since the two conformations, S-cis and S-trans, rapidly interconvert. This reaction occurs in a single step, requiring the diene to adopt an S-cis conformation for simultaneous bonding of its end carbon atoms (#1 & #4) with the dienophile. However, certain dienes possess extreme steric hindrance, making the S-cis conformation highly strained. Consequently, such dienes are not readily reactive in Diels-Alder reactions.
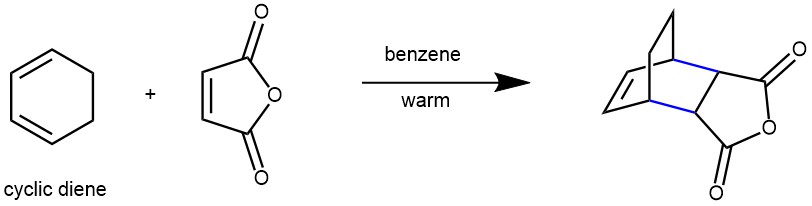
On the other hand, cyclic dienes are locked into the S-cis conformation, making them particularly reactive in these reactions. When a cyclic diene participates in a Diels-Alder reaction, it results in the formation of a bicyclic structure.
DIENOPHILE (diene lover): Ethylene is the simplest dienophile. The Diels-Alder reaction proceeds rapidly with electron-withdrawing groups. Propenal, ethyl propenoate, acrylonitrile, maleic anhydride, etc. are highly reactive in the Diels-Alder reaction.
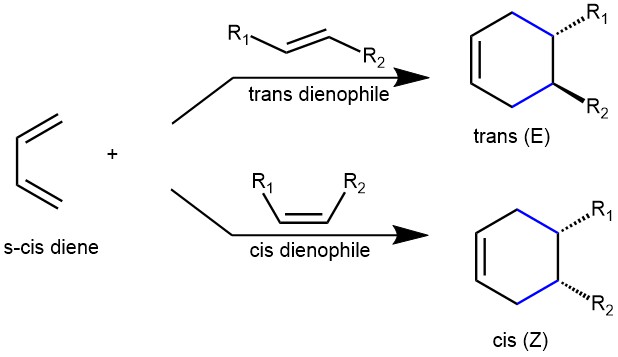
STEREOCHEMISTRY OF THE DIELS-ALDER REACTION: The stereochemical information (E or Z) in the diene is transferred to the product. If a disubstituted cis (Z) alkene is used, the stereochemistry of the two substituents in the product will be cis and when a trans (E) alkene is used, the stereochemistry in the product will be trans. The retention of stereochemistry is due to the planar nature of both reactants and because the addition is suprafacial (i.e., to or from the same face of each plane) or synaddition.
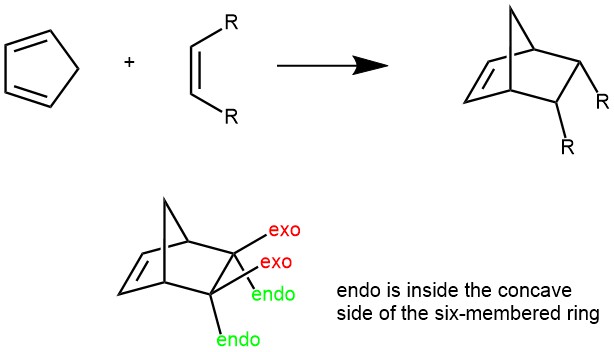
The Diels-Alder reaction predominantly occurs in the endo orientation rather than the exo orientation. This means that any additional unsaturated groups present in the dienophile (e.g., -CO-O-CO- group in maleic anhydride) tend to be positioned close to the developing double bond in the diene component. In the context of bicyclic structures like substituted norbornanes, the terms endo and exo refer to the relative stereochemistry. The endo position is situated inside the concave shape of the larger six-membered ring, while the exo position is located on the outside.
When cyclic dienes participate in Diels-Alder reactions, they tend to favor the formation of bicyclic structures where substituents are oriented in the endo position. This orientation is preferred because it enhances the required orbital overlap between the diene and dienophile, promoting a more favorable reaction.
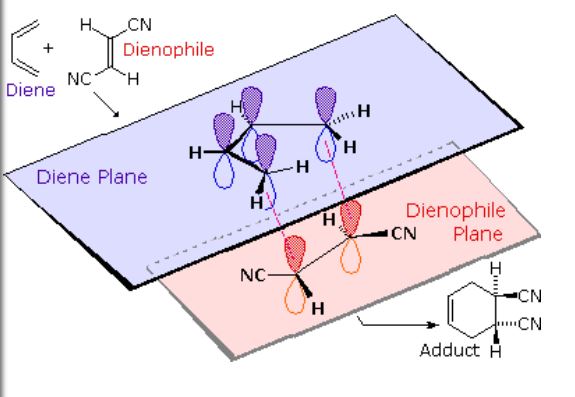
MECHANISM: The Diels-Alder reaction is a pericyclic reaction, meaning it involves a concerted movement of electrons through a cyclic transition state. It is classified as a [π4s + π2s] cycloaddition, indicating that it proceeds through the suprafacial/suprafacial interaction of a 4π electron system (the diene structure) with a 2π electron system (the dienophile structure). The driving force behind the reaction is the cleavage of weak π-bonds and the formation of two new stronger σ-bonds.
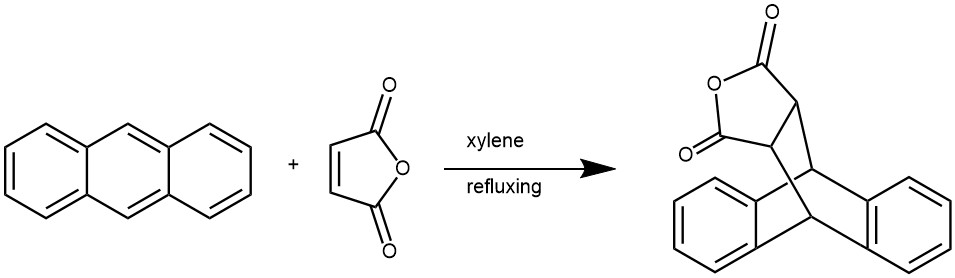
EXAMPLE 1: About 5.0 g (0.028 mol) anthracene, 2.75 g (0.028 mol) maleic anhydride and 52 ml xylene were added to a 100 ml three-neck boiling flask. The reaction mixture was refluxed for 2 h under stirring, then cooled to room temperature. The product crystallized under ice-water bath for 30 min, then filtrated off and washed with several milliliters of ethanol. The pure product was obtained as a white crystals. m.p. 265–266 °C, 82%.[REF: Journal of Organometallic Chemistry 694 (2009) 2981–2986]
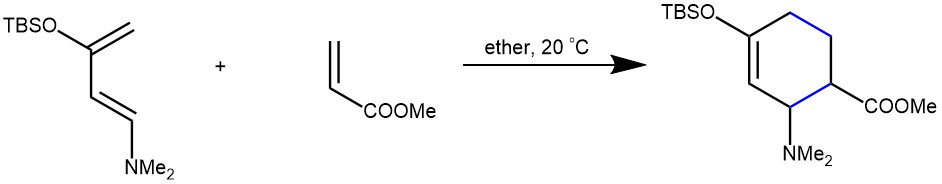
EXAMPLE 2: A dry, 250-mL, round-bottomed flask is charged with 1-dimethylamino-3-tert-butyldimethylsiloxy-1,3-butadiene (16.6 g, 0.073 mol) and anhydrous ether (30 mL). Methyl acrylate (13.5 mL, 0.15 mol) is added in one portion and the resulting light yellow solution is allowed to stand for 20 hr at room temperature. The solution is concentrated on a rotary evaporator, and the residual methyl acrylate is removed under a high vacuum to afford 22.4 g (98%) of the desired cycloadduct 2, in good purity, as a pale-yellow oil. [REF: Organic Syntheses, Coll. Vol. 10, p.442 (2004); Vol. 78, p.160 (2002)]
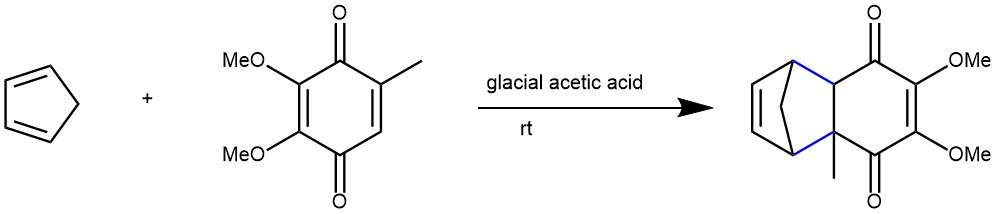
EXAMPLE 3: To a solution of 2,3-dimethoxy-5-methyl-p-benzoquinone (4.0 g, 21.96 mmol) in glacial acetic acid (100 mL) was added freshly distilled cyclopentadiene (2.8 mL, 32.94 mmol, 1.5 eq) and the reaction was stirred overnight at r.t. The reaction was cooled to 0 °C and ice/water was added. The aqueous layer was neutralized using 3M aq. NaOH and extracted with ethyl acetate (3 × 50 mL). The combined organic layers were washed with water, and brine, dried on sodium sulfate, filtered, and evaporated affording the title compound (5.4 g, yield: 98%) as a dark red oil. [REF: NICOZ S.A. – WO2013/60673, 2013, AI]
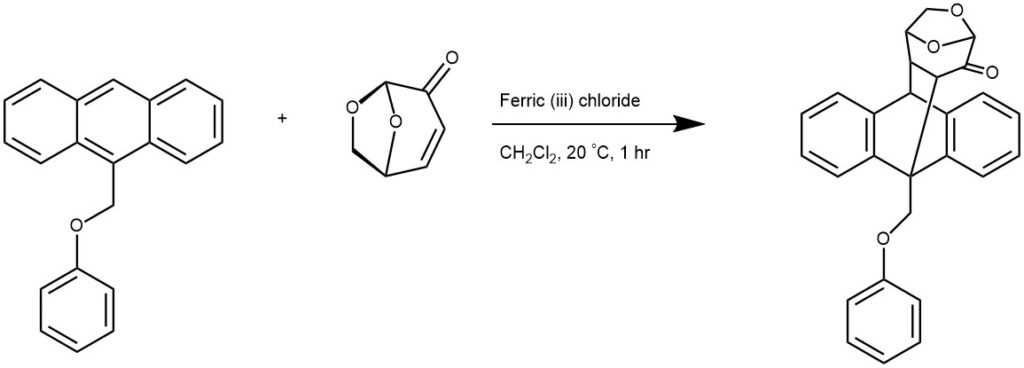
EXAMPLE 4: To a solution of levoglucosenone (470 mg, 3.73 mmol) in dry dichloromethane (30 mL) was added 9-methoxyanthracene 2b (1010 mg, 4.85 mmol) and anhydrous FeCl3 (121 mg, 0.75 mmol). The mixture was stirred at room temperature for 1h. The reaction was diluted with dichloromethane and washed with water (50 mL). The organic layer was separated and the aqueous phase was extracted with dichloromethane (4 × 70 mL). The combined organic layer were dried over anhydrous sodium sulfate and concentrated. Purification by flash chromatography furnished the product (908 mg, 2.71 mmol, 73% yield) as a yellow crystalline solid. [REF: Tetrahedron Letters, 2014, 55, 42, 5832-5835]
REFERENCES:
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako
- Diels–Alder reaction. (2023, June 21). In Wikipedia. https://en.wikipedia.org/wiki/Diels%E2%80%93Alder_reaction
- https://chem.libretexts.org/