Dieckmann condensation is a type of organic reaction that involves the intramolecular condensation of diesters with base to form cyclic β-ketoesters. The reaction is named after the German chemist Walter Dieckmann. Dieckmann condensation was first reported by Walter Dieckmann in 1894, who synthesized cyclic β-ketoesters from α,ω-diesters using potassium cyanide as a base. Later, he also used sodium ethoxide and sodium methoxide as bases for the same reaction. The equivalent intermolecular reaction is the Claisen condensation. Thus, the base mediated condensation of an ester, containing an α-hydrogen atom, with a molecule of the same ester to give a β-ketoesters is known as the Claisen condensation. When the two reacting ester functional groups are present in the same molecule, it is called a Dieckmann condensation. The Dieckmann condensation forms 5-, 6-, 7-, and 8-membered rings in high yield but low yields are obtained for larger rings. However, with high-dilution method larger rings are possible.
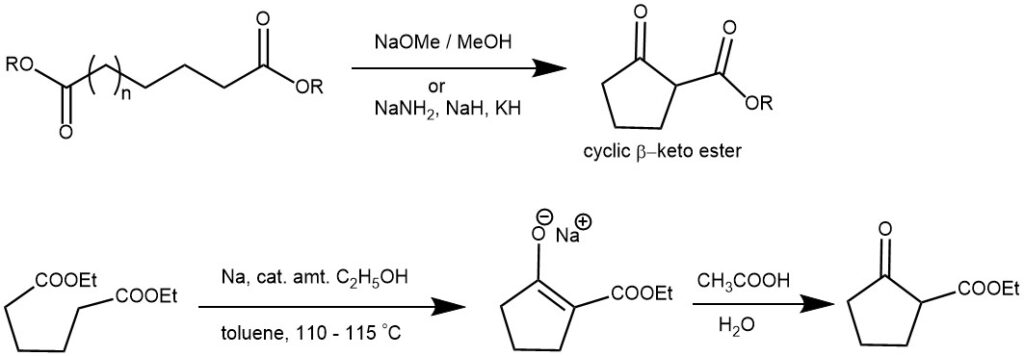
MECHANISM: The reaction mechanism involves the deprotonation of an ester at the α-position by the base, generating an enolate ion. The enolate ion then undergoes a 5-exo-trig or 6-exo-trig nucleophilic attack to the other ester group in the same molecule, forming a cyclic enol. Protonation of the enol with an acid re-forms the β-ketoester. The driving force of the reaction is the generation of the resonance-stabilized enolate of the product β-ketoesters. Also, the condensation usually fails if it is not possible to generate this stable intermediate.
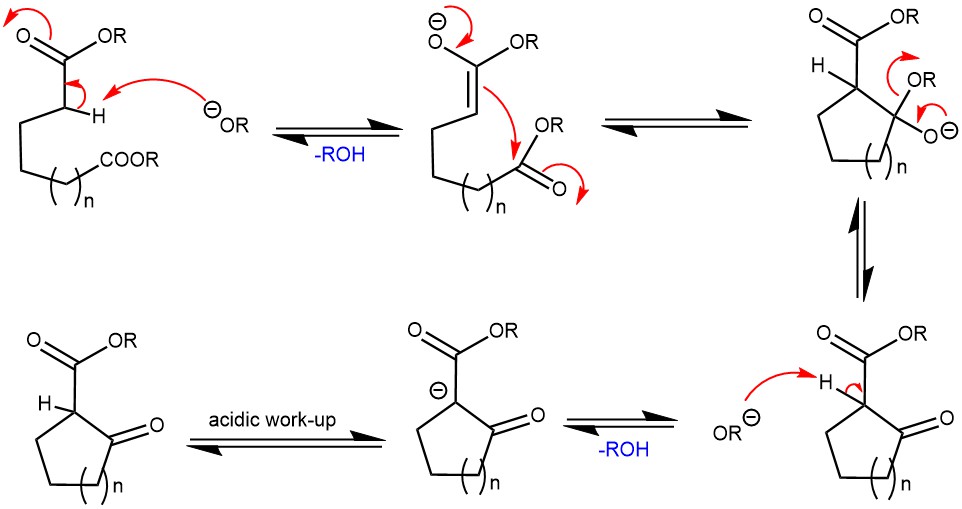
EXAMPLE 1: Starting compound 2a (600 mg, 1.30 mmol, 1 equiv) was dissolved under argon in freshly distilled THF (4 mL). To this solution was added a molar solution of TBAF in THF (3.9 mL, 3.9 mmol, 3 equiv) and the mixture stirred at r.t. overnight. Aq 6 N HCl (20 mL) and Et2O (20 mL) were then added to the mixture. The mixture was concentrated and precipitated with CH2Cl2 at 0 °C, affording compound 3a as a white powder; yield: 437 mg (85%).[REF: Synthesis 2010, No. 10, 1697–1701]
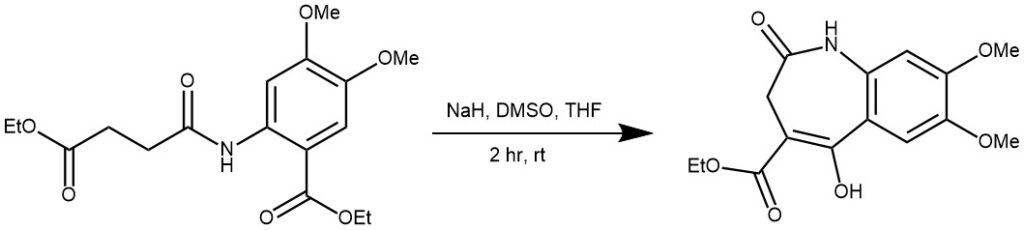
EXAMPLE 2: To a suspension of NaH (5.95 g, 60%, deoiled) in DMSO (100 mL) and THF (15 mL) at room temperature a solution of the amide (25 g, 70.75 mmol) in DMSO (100 mL) was added dropwise over 1 h and the mixture stirred for 1 h. A cooled solution (0 C) of HOAc (40 mL) was added, and the resulting solution was stirred for 30 min. H2O (250 mL) was added drop-wise and the resulting white precipitate filtered off, dissolved in CH2 Cl2 /MeOH (90:1), washed with brine, dried (MgSO4), and concentrated. The resulting residue was stirred with n-pentane and diethyl ether to afford a white amorphous solid: 19.5g, 89.7%.[REF: Bioorg. Med. Chem. 11 (2003) 1319–1341]
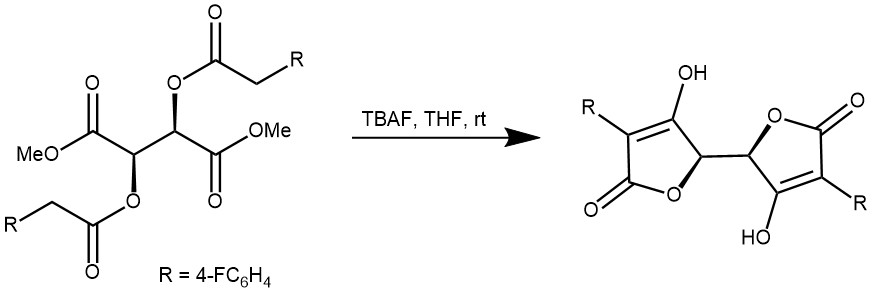
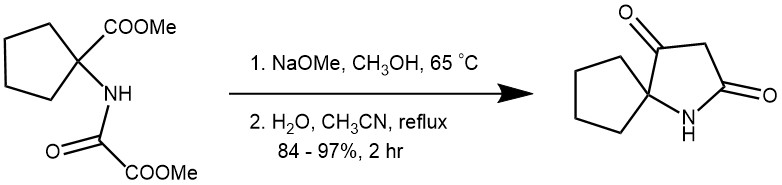
EXAMPLE 3: The starting material (34.7 g, 0.135 mol, 1 equiv.) was dissolved in dry methanol (200 mL). A solution of NaOMe (16.2 g, 0.3 mol, 2.2 equiv.) in dry methanol (100 mL) was added to the mixture at r.t. The resulting mixture was stirred at 65 °C overnight. The solvent was removed under reduced pressure. The residue was dissolved in water (200 mL), acidified with 1 M HCl and extracted with CH2Cl2 (2 × 300 mL). The combined organic fractions were dried with Na2SO4 and concentrated under reduced pressure. The resulting residue was dissolved in CH3CN (300 mL). Distilled water (9.7 mL, 0.54 mol, 4 equiv.) was added to the mixture. The mixture was refluxed for 2 hours and concentrated under reduced pressure. The crude product was solidified in MTBE, filtered, and washed with hexane to give pure product.[REF: Eur. J. Org. Chem. 2019, 3553–3559]
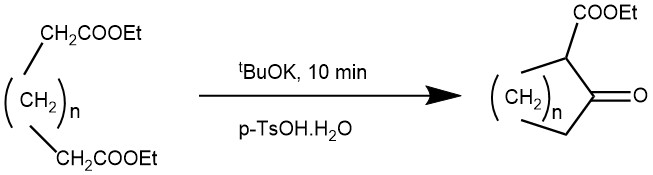
EXAMPLE 4: When 1a (10.2 g, 50.4 mmol) and ButOK powder (8.44 g, 75.2 mmol) were mixed using a mortar and pestle for 10 min, the reaction mixture solidified. The solidified mixture was kept in a desiccator for 60 min. The reaction mixture was neutralized by addition of p-TsOH.H2O and was distilled under 20 mmHg to give 2a (5.8 g, 82% yield)[REF: J. Chem. Soc., Perkin Trans. 1, 1998, 3521–3522]
REFERENCES:
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako
- Modern synthetic reactions by Herbert O. House


