The Darzens reaction or Darzens glycidic ester condensation is a chemical reaction of a ketone or an aldehyde with an α-halo ester in the presence of a base to form an α,β-epoxy ester, also called “glycidic ester”. The reaction was discovered by the organic chemist Auguste Georges Darzens in 1904. The most frequently used bases are potassium ter-butoxide, sodium ethoxide, sodium amide, LDA, etc. The reaction is general, since aromatic aldehydes and ketones, aliphatic ketones as well as α,β-unsaturated, and cyclic ketones react smoothly and give good yields of the product. Glycidic esters are versatile synthetic intermediates as the epoxide group can be opened with various nucleophiles.
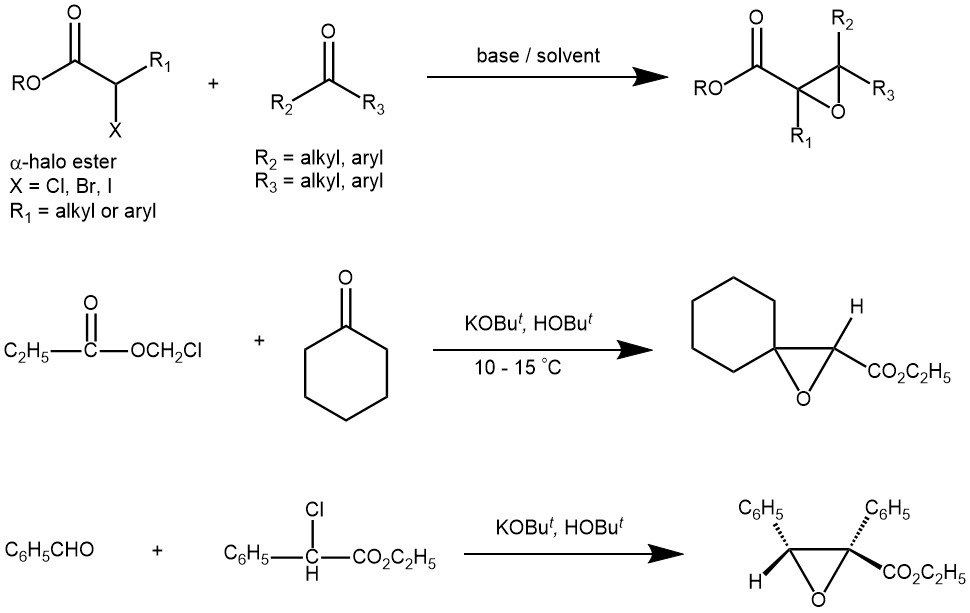
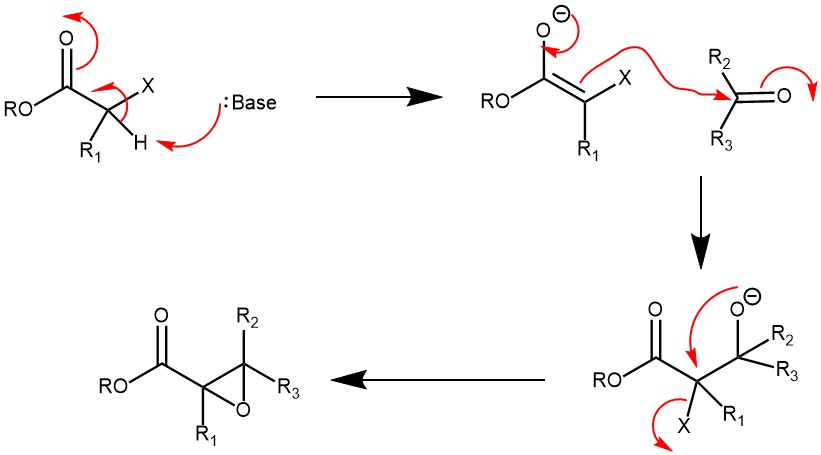
MECHANISM: The mechanism is very similar to an Aldol reaction. The base deprotonates an α-hydrogen from the α-halo ester forming a carbanion (enolate). The anion then attacks the carbonyl carbon of the aldehyde/ketone forming a tetrahedral intermediate that undergoes SNi reaction to form the product.
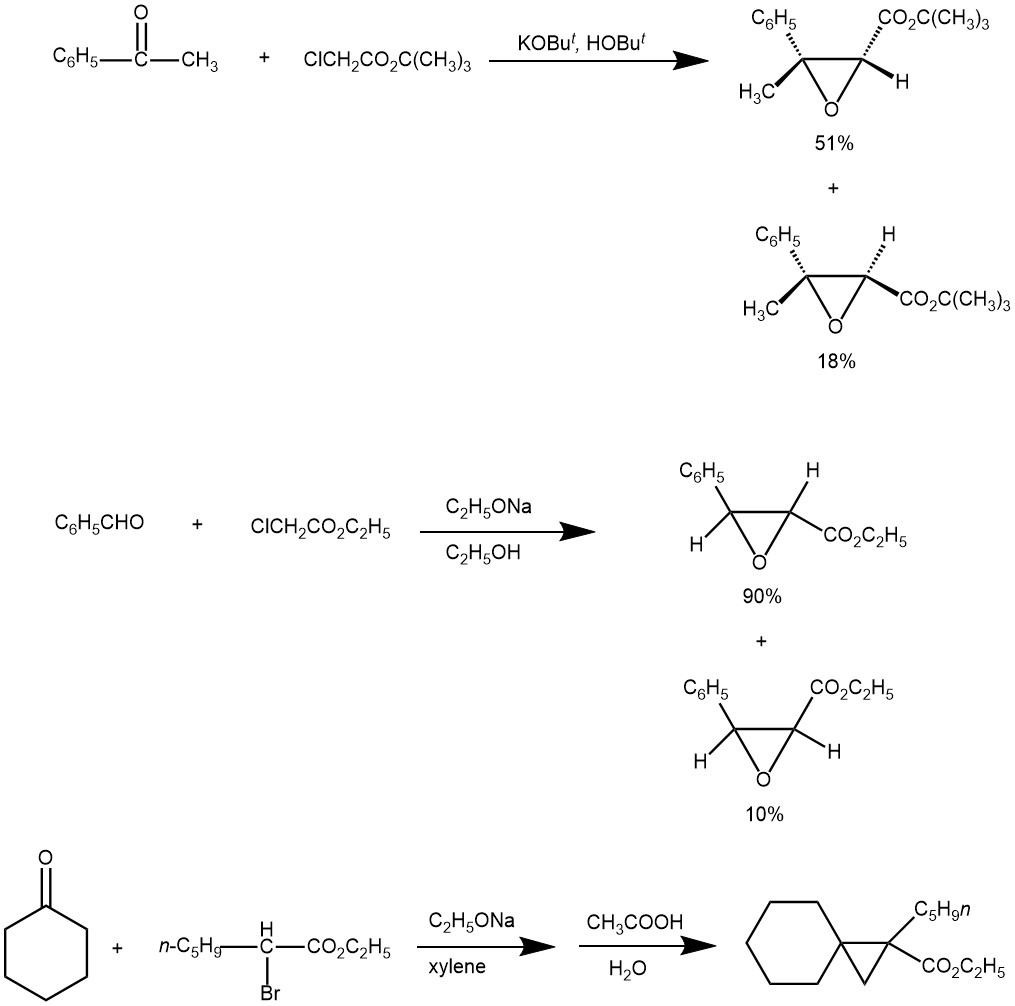
SOME EXAMPLES
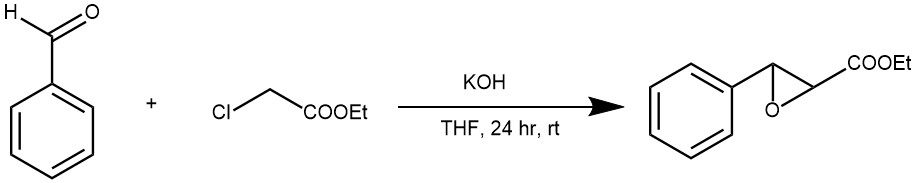
EXAMPLE 1: In a typical Darzens condensation, a mixture of aldehyde (5.0 mmol), α -chloroester (6.0 mmol) and catalyst (2 mol %) was suspended in THF (10 ml) and stirred for 30 min. Base (6.0 mmol) was added to the above reaction mixture. After stirring for a stipulated period (24 hours) at room temperature, the reaction was quenched with water and was extracted with ethyl acetate (3×15 ml). The combined organic layer was washed with brine and solvent, dried with anhydrous sodium sulfate, and concentrated under reduced pressure. Following purification by flash column chromatography, the isolated yield was obtained [REF: Tetrahedron Letters 48 (2007) 4489–4493].
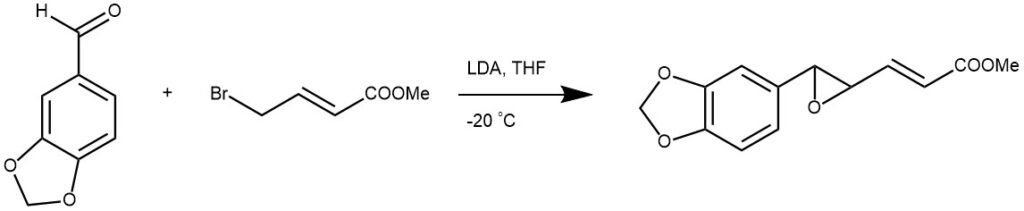
EXAMPLE 2: LDA (34mmol) {generated by the addition of n-BuLi 1.6M (23.4 ml, 37.4 mmol) to a solution of diisopropylamine (4.75 ml, 34 mmol) in THF (40 ml) at –20°C under N2 } was added dropwise to a stirred solution of piperonal (10.2g, 68 mmol) and methyl 4-bromocrotonate (2 ml, 17 mmol) in THF (60 ml) under N2 at –20°C. Typically, the transfer lasted 1hr for 20 mmol of crotonate. The reaction was stirred for 2h at –20°C and then quenched with sat. aq. NH4Cl (40ml). The layers were separated and the aqueous layer was extracted with ether (3×20 ml). The combined organic layers were then washed with sat. aq. NaHSO3 (40g of NaHSO3 solid), sat. aq. NaHCO3 (20 ml) and brine (3×30 ml), dried (MgSO4 ) and concentrated. Purification by flash chromatography (ether : petrol (1:3)) afforded the title ester as (43:57) mixture of syn and anti epoxides (2.95g, 70%)[ REF: . Org. Lett., Vol. 4, No. 7, 2002]
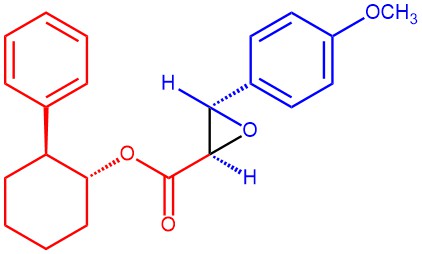
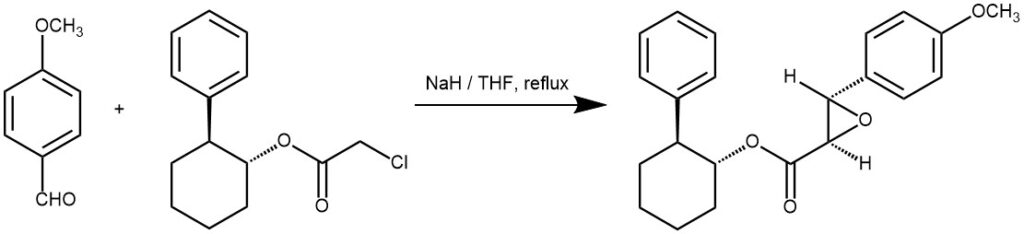
EXAMPLE 3: A 5-L three-necked flask equipped with a stirrer, a gas bubbler, a thermometer, an addition funnel, and a condenser was charged with 100 g (3.33 mol) of NaH (80% in mineral oil) and triturated with three 500-mL portions of hexanes. Under an argon atmosphere, 1.5 L of THF was added followed by the addition of a solution of 568 g (2.247 mol) of (-)-irons-2-phenylcyclohexyl chloroacetate ((-)-10a) and 352 g (2.589 mol) of anisaldehyde in 250 mL of THF over 0.5 h via an addition funnel. A vigorous evolution of H2 gas ensued along with an exotherm to ~55 °C. After the initial reaction subsided, the mixture was then stirred under argon at ambient temperature overnight The mixture was heated at 55-60 °C for 1 h and poured into 8.0 L of ice water. The pH was adjusted to 7 (pH meter) with 390 mL of 3 N HgSOa, and the resulting mixture was extracted first with 4 L followed by 2 X 2 L of CH2C12. The combined organic layers were dried (Na^Oa) and evaporated in vacuo to an oily, partially solid residue, which on crystallization from 0.5 L of (9:1) hexanes-EtOAc afforded colorless needles. These crystals were collected by filtration, washed with 2 X 200 mL of 9:1 hexanes-EtOAc followed by 2 X 500 mL of cold hexanes, and finally dried in vacuo to give 415 g (52%) of the product [REF: J. Org. Chem., Vol. 57, No. 3, 1992, 852-856].
REFERENCES;
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako
- Modern synthetic reactions by Herbert O. House