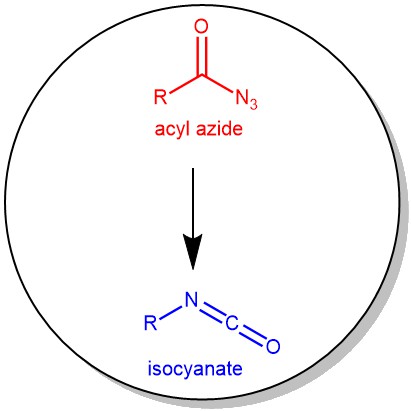
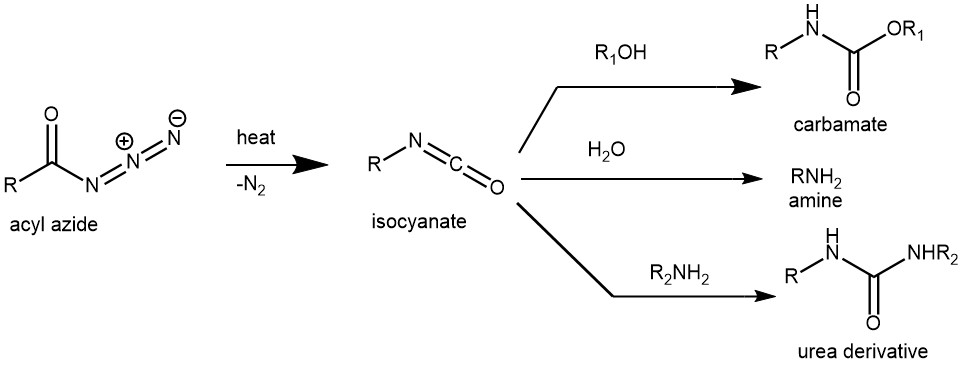
The thermal decomposition of acyl azides to the corresponding isocyanates is known as the Curtius rearrangement. This rearrangement is named after the German chemist Theodor Curtius, who first observed this transformation in his studies on azides in 1885.
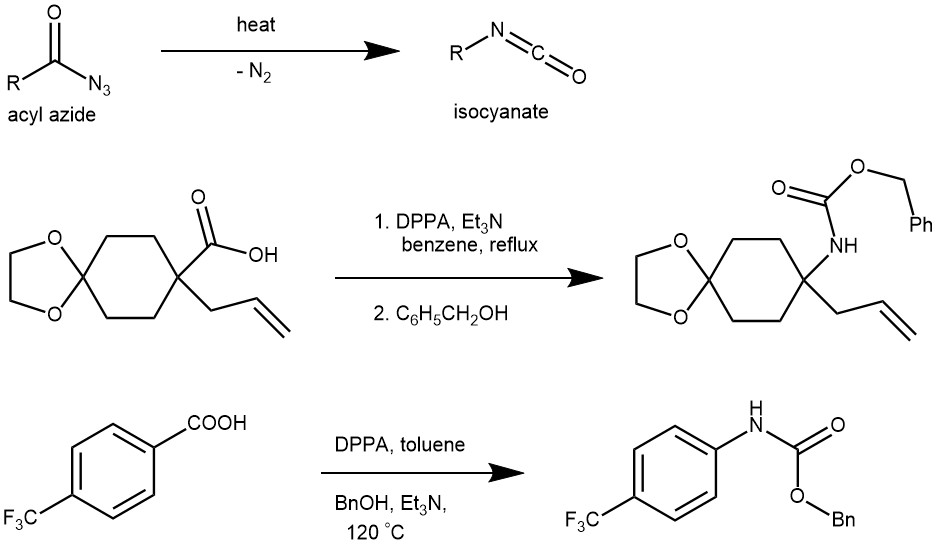
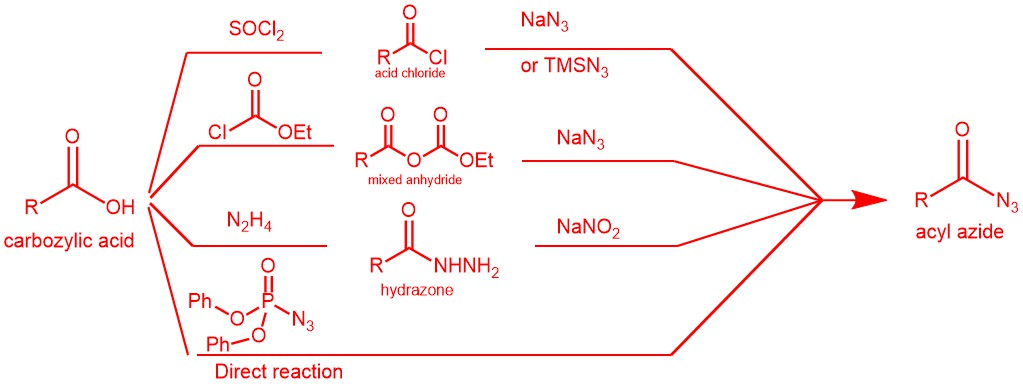
The acyl azides necessary for this transformation (rearrangement) can be prepared from the reaction of acid chlorides or acid anhydrides with sodium azide or trimethylsilyl azide. It can also be obtained by treating acyl hydrazines with nitrous acid or nitrosonium tetrafluoroborate. More importantly, acyl azide can be obtained by the direct reaction of a carboxylic acid with diphenyl phosphoryl azide (DPPA).
The Curtius rearrangement finds extensive applications in organic synthesis due to its ability to introduce isocyanate functional groups. Isocyanates are crucial intermediates for synthesizing pharmaceuticals, agrochemicals, and polymers. They also serve as precursors for the synthesis of ureas, carbamates, and other nitrogen-containing compounds. The isocyanates react with a variety of nucleophiles such as water, alcohols, and amines to liberate primary amine, carbamate, or urea derivatives respectively.
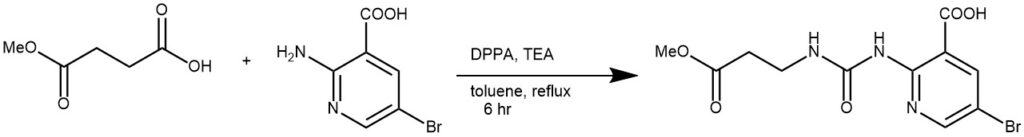
EXAMPLE 1: To a solution of A (14.6 g, 110.6 mmol) in toluene (530 mL) was added DPPA (30.4 g, 110.5 mmol), followed by TEA (18.3 g, 181.2 mmol). The mixture was stirred at RT for 30 min, then B (15.9 g, 73.61 mmol) was added. The reaction mixture was stirred at reflux for 6 h. The mixture was diluted with EtOAc (530 mL) and washed with brine (2 x 300 mL). The combined aq layers were acidified to pH 5 using 2N HCl, causing solids to form. The resulting solids were filtered and dried to provide the product as a pale yellow solid. [15 g, 39.3%][REF: World Intellectual Property Organization WO2016023830, page 60]
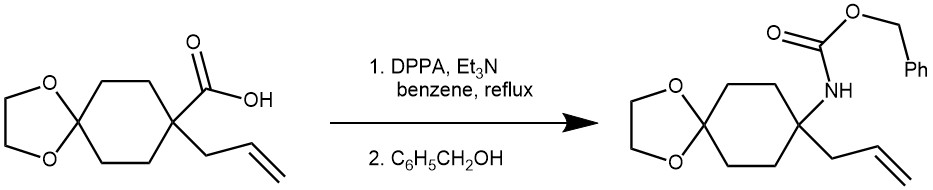
EXAMPLE 2: A mixture of the acid (5.00 g, 22.1 mmol), triethylamine (7.7 ml, 55.3 mmol), diphenylphosphoryl azide (7.2 ml, 33.2 mmol), and benzene (70 ml) was refluxed for 30 min. After addition of benzyl alcohol (22.9 ml, 221 mmol) at room temperature, the reaction mixture was further refluxed for 5 h and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel eluted with hexane/AcOEt, 5:1) to give the product 4.72 g, 14.3 mmol, 65%) as a colorless oil.[REF: Tetrahedron 58, 2002) 4917-4924]
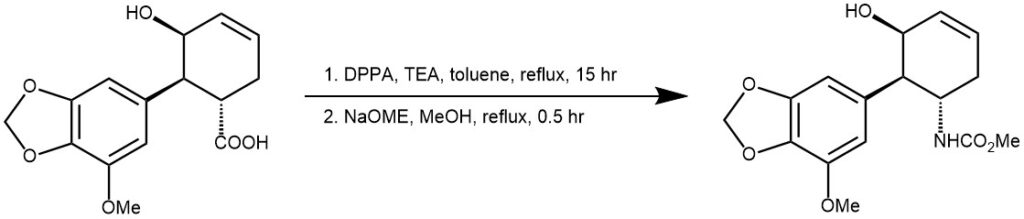
EXAMPLE 3: The mixture of carboxylic acid (1.04 g, 3.6 mmol), triethylamine (1.49 mL, 10.7 mmol) and diphenylphosphoryl azide (2.3 mL, 10.6 mmol) in toluene (42 mL) was stirred at room temperature for 20 min until the solution was clear. This reaction mixture was heated slowly to reflux for 15 h and cooled to room temperature. Then the mixture was diluted with EtOAc. It was washed with saturated aqueous NH4Cl, brine and dried over Na2SO4. This crude product was evaporated and chromatographed on silica gel column in Hexane/EtOAc (3:2) to give isocyanate intermediate (IR 2442, 1691 cm–1 ). This intermediate was dissolved in MeOH (40 mL) and added NaOMe (0.33 mg, 6.1 mmol). The reaction mixture was heated to reflux for 30 min, then cooled to room temperature. The reaction was quenched with saturated aqueous NH4Cl and diluted with EtOAc. It was washed with brine, dried over Na2SO4 and evaporated under reduced pressure. The resulting residue was purified by column chromatography (hexanes/EtOAc = 3:2) to give colorless syrup 13 (938 mg, 82%).[REF: Org. Lett., Vol. 4, No. 8, 2002]
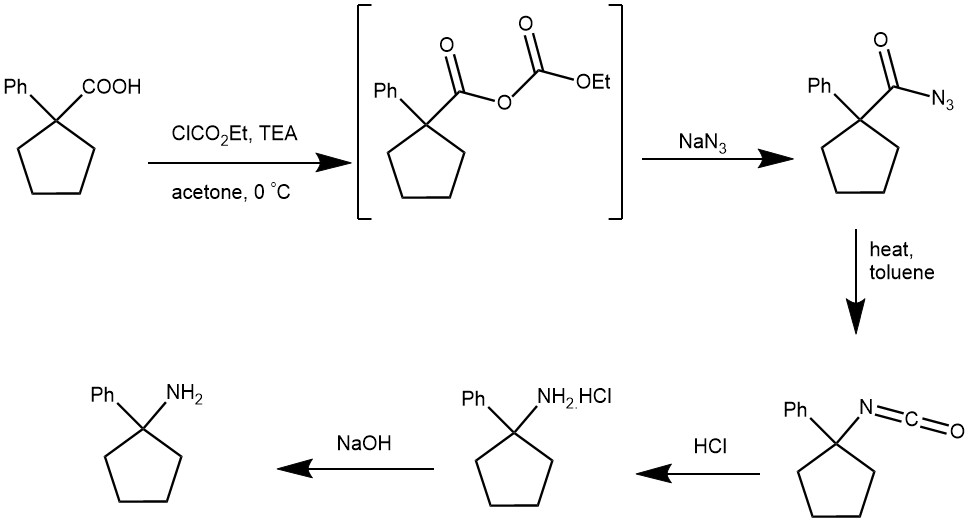
EXAMPLE 4: A 1L three-necked, round-bottomed flask equipped with an air stirrer, dropping funnel, and low temperature thermometer is charged with 38.0 g. (0.200 mole) of 1-phenylcyclopentanecarboxylic acid and 150 ml. of acetone. The mixture is stirred, and 22.3 g. (30.6 ml., 0.221 mole) of triethylamine is added over 5 minutes (a 2° rise in temperature is observed). The solution is chilled to −5 to 0° in an ice-salt bath, and 24.0 g. (21.1 ml., 0.221 mole) of ethyl chlorocarbonate in 50 ml. of acetone is added slowly (25 minutes), maintaining the temperature between −5 to 0°. After the addition is complete, the cold mixture is stirred for an additional 15 minutes. A solution of 26.0 g. (0.400 mole) of sodium azide in 75 ml. of water is added over a 25-minute period while the temperature is kept at −5 to 0°. The mixture is stirred for 30 minutes longer at this temperature, poured into 750 ml. of ice water, and shaken with four 250-ml. portions of toluene. The combined toluene extracts are dried over anhydrous magnesium sulfate and transferred to a 2L., three-necked, round-bottomed flask equipped with a two-necked, Claisen-type adapter, stirrer, and reflux condenser. The stirred solution is heated cautiously under reflux for 1 hour on a steam bath (nitrogen evolution is observed initially). The toluene is then removed at 50° with a rotary evaporator (aspirator pressure). The flask containing the residual, oily isocyanate is again fitted with the Claisen-type adapter, stirrer, and reflux condenser. The oil is stirred, cooled in an ice bath, and 300 ml. of 8 N hydrochloric acid is added. The cooling bath is removed, the stirred mixture is gradually heated on a steam bath until carbon dioxide evolution has subsided (30 minutes), and the solution is heated under reflux for 10 minutes. The flask is evacuated with a water aspirator and warmed in a bath at 50° for about 10 minutes. About 10–20 ml. of distillate is collected before a crystalline product separates. Ice water (200 ml.) is added to the flask with cooling in an ice bath, and 1 l. of 2.5 N sodium hydroxide is added slowly to pH 12. The mixture is shaken with three 200-ml. portions of diethyl ether, the combined extracts are dried over anhydrous magnesium sulfate, and the ether is removed by distillation at 50° with a water aspirator, yielding 29.7 g. of an oil. Distillation of the crude product gives 24.5–26.1 g. (76–81%) of 1-phenylcyclopentylamine, b.p. 112–114° (9 mm.). [REF: Organic Syntheses, Coll. Vol. 6, p.910 (1988); Vol. 51, p.48 (1971)]