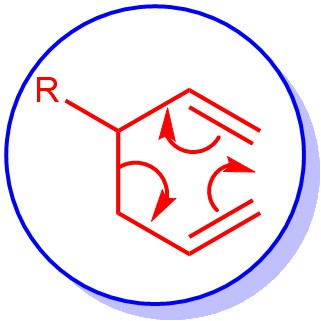
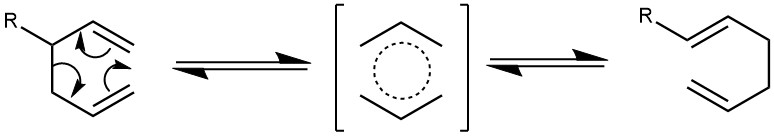
The thermal [3,3]-sigmatropic rearrangement of 1,5-dienes to the regioisomeric 1,5-dienes is called the Cope Rearrangement. The main product is the thermodynamically more stable regioisomer.
The reaction is reversible, and the reaction is shifted toward the forward direction (on the product side) if the product is more stable due to stabilization by conjugation or the double bond is more highly substituted than the reactant. The typical temperature required to induce cope rearrangement in acyclic dienes is 150-260 °C. However, the reactions can be achieved at milder conditions when the dienes are substituted in the C3 or C4 position or the dienes are cyclic and ring strain is relieved during the reaction or by using a catalyst.
The Cope rearrangements of divinyl cyclopropanes are particularly interesting because they are facile at relatively low temperatures due to the rigidity of the system and the strain release which provides a thermodynamic driving force
MECHANISM: It is classified as a [3,3]-sigmatropic rearrangement. It proceeds via a chair-like transition state where there is minimal steric interaction between the substituents.
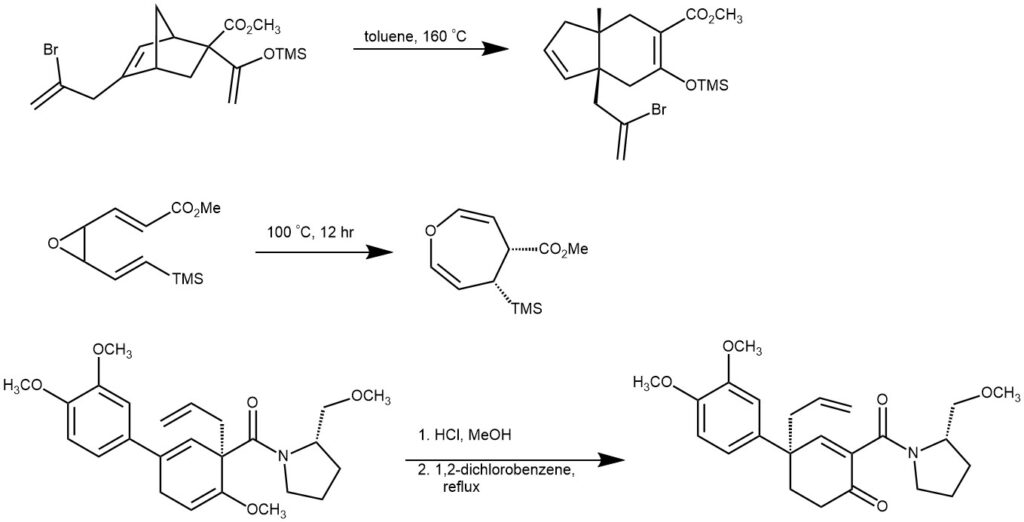
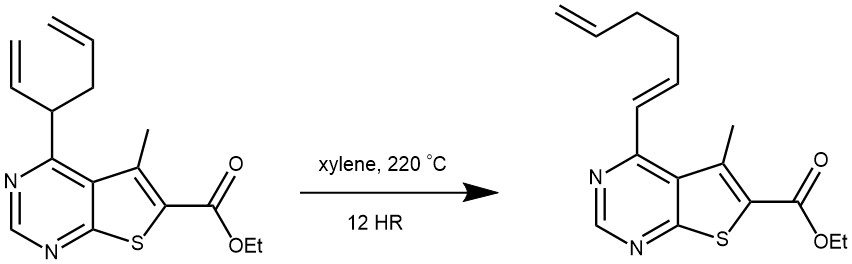
EXAMPLE 1: Ethyl 4-(hexa-1,5-dien-3-yl)-5-methylthieno[2,3-d]pyrimidine-6-carboxylate (200 mg, 0.66 mmol) was dissolved in xylene and heated with stirring in a sealed tube at 220°C for 12 h. TLC analysis (eluent hexane–EtOAc, 9:1) indicated full conversion of the starting material. The reaction mixture was concentrated at reduced pressure, and the desired compound was isolated chromatographically on silica gel using hexane–EtOAc, 1:0 to 9:1, as a mobile phase. Yield 150 mg (75%), brown solid, mp 51–52°C.[REF: Chemistry of Heterocyclic Compounds 2019, 55(12), 1269–1273]
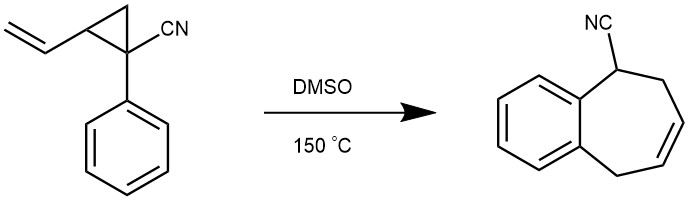
EXAMPLE 2: A 100 mg/mL solution of vinyl cyclopropane in DMSO was prepared, and 0.5 to 1 mL of this solution was added to a flame dried vial equipped with a stir bar and diluted to 0.2 M with DMSO. The vial was sealed and the atmosphere was replaced with argon by purging and refilling with argon 3×. The mixture was heated to 150 °C and monitored via NMR. Once the reaction was complete (generally 1–6 hours), the mixture was diluted with ethyl acetate (2 mL) and washed with water (5 mL). The first wash was back extracted with ethyl acetate (1mL), and the combined organic extracts were washed again with water (5 mL) and brine (1 mL). The organic layer was then dried with MgSO4, filtered, and the solvents were evaporated to afford the non-conjugated benzocycloheptene. For most products, no further purification was needed. In some cases, additional purification via flash chromatography was required.[REF: Tetrahedron, 2019, 75, 24, 3319-3329]
REFERENCES:
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako