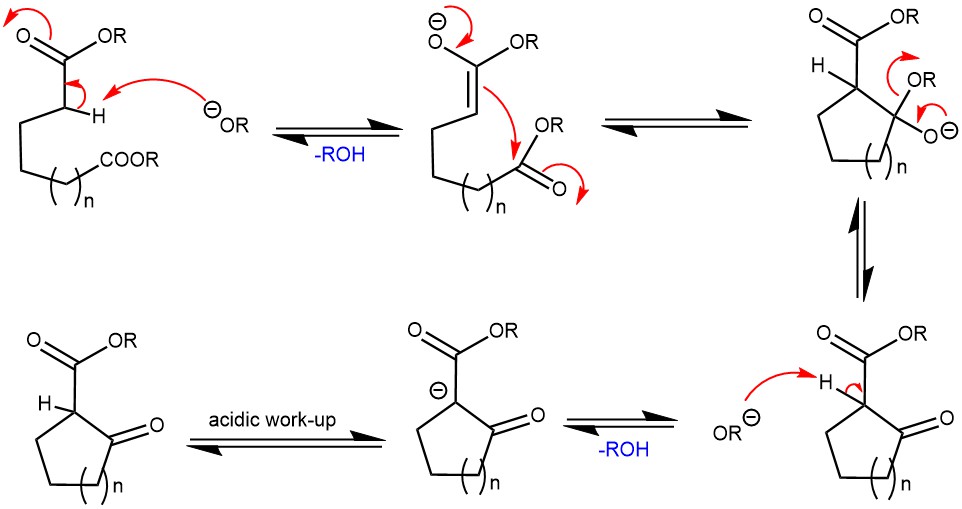
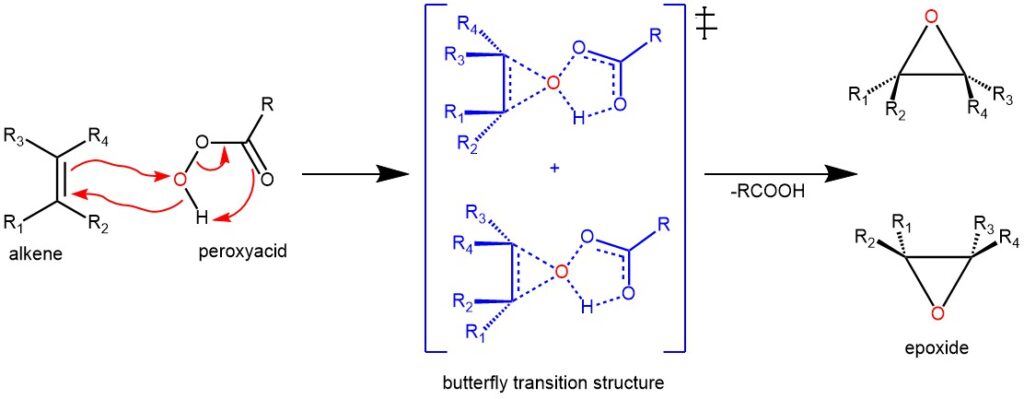
DIECKMANN CONDENSATION: FORMATION OF CYCLIC βeta-KETOESTERS
Dieckmann condensation is a type of organic reaction that involves the intramolecular condensation of diesters with base to form cyclic β-ketoesters. The reaction is named after the German chemist Walter Dieckmann. Dieckmann condensation was first reported by Walter Dieckmann in 1894, who synthesized cyclic β-ketoesters from α,ω-diesters using potassium cyanide as a base. Later, he …
DIECKMANN CONDENSATION: FORMATION OF CYCLIC βeta-KETOESTERS Read More »