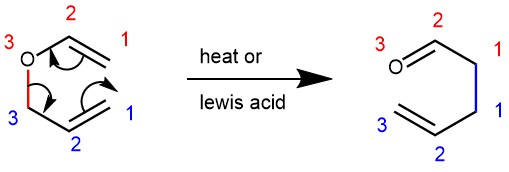
MEERWEIN-PONNDORF-VERLEY REDUCTION: REDUCTION OF ALDEHYDES AND KETONES TO ALCOHOLS
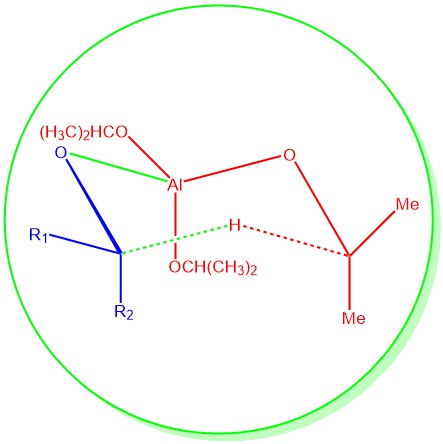
The reduction of aldehydes and ketones to the primary and secondary alcohols represents important synthetic transformations. One of the most chemoselective and mild reactions for these types of reductions is the Meerwein-Ponndorf-Verley (MPV) reductions. It is the reduction of aldehydes and ketones to the corresponding alcohols using aluminum alkoxide (usually aluminum isopropoxide) in the presence …
MEERWEIN-PONNDORF-VERLEY REDUCTION: REDUCTION OF ALDEHYDES AND KETONES TO ALCOHOLS Read More »