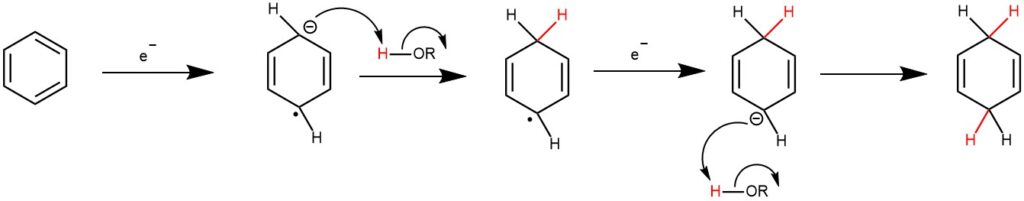
The 1,4-reduction of aromatic rings to the corresponding unconjugated cyclohexadiene by alkali metals (Li, Na, K) dissolved in liquid ammonia in the presence of an alcohol is called the Birch reduction. Although the relative rates of reduction with the alkali metals and ethanol in liquid ammonia follow the order Li > Na > K, all three are sufficiently rapid to be synthetically useful.
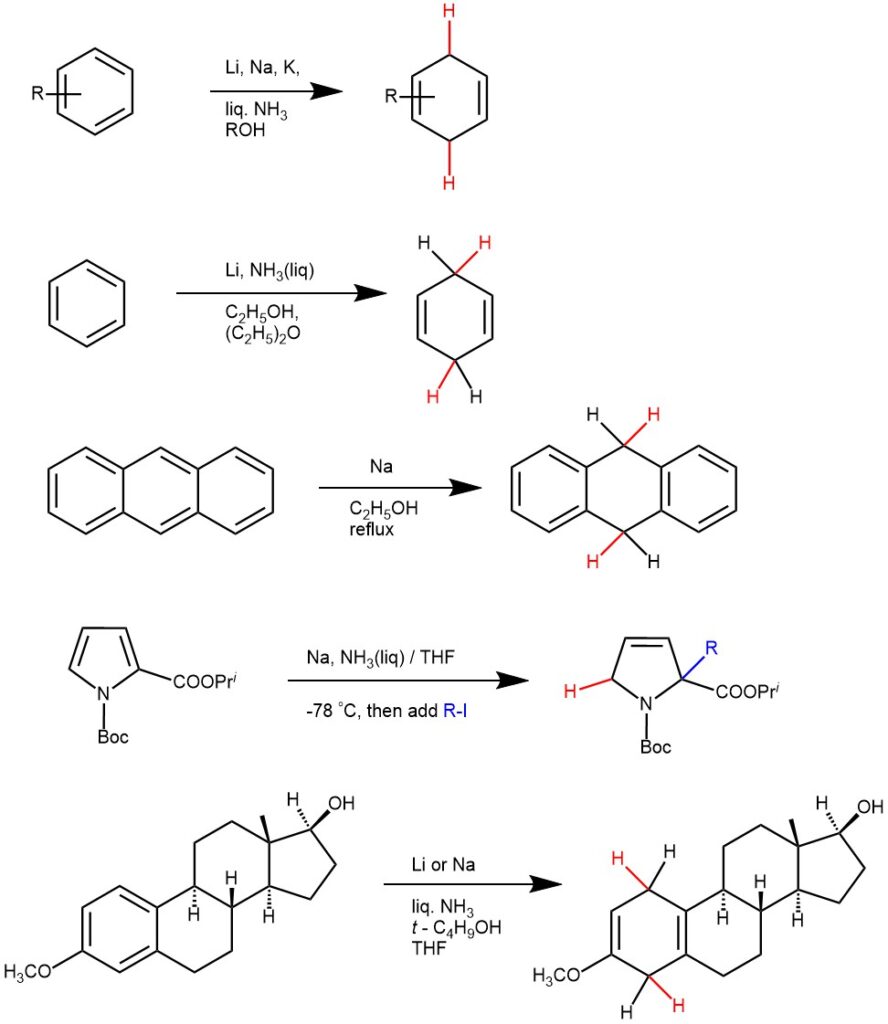
In some cases, solubility issues can be encountered. This problem of the insolubility of the aromatic compounds in the reaction medium (alcohol in liq. Ammonia) is overcome by the use of co-solvents such as ether, THF, EtOH, t-BuOH, etc.
Substituents that can aid in charge delocalization or electron-withdrawing groups on an aromatic system will favor the acceptance of an electron to form an anion radical and accelerate the reduction. The dihydrobenzenes produced from benzene derivatives having electron-withdrawing substituents have been found to be 1-substituted-1,4-dihydrobenzenes.
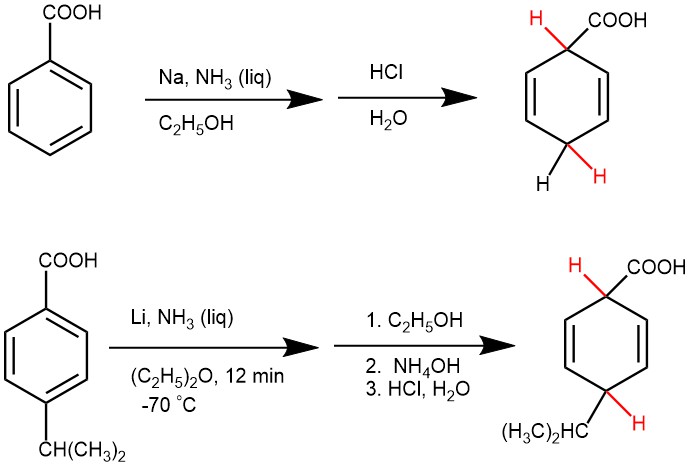
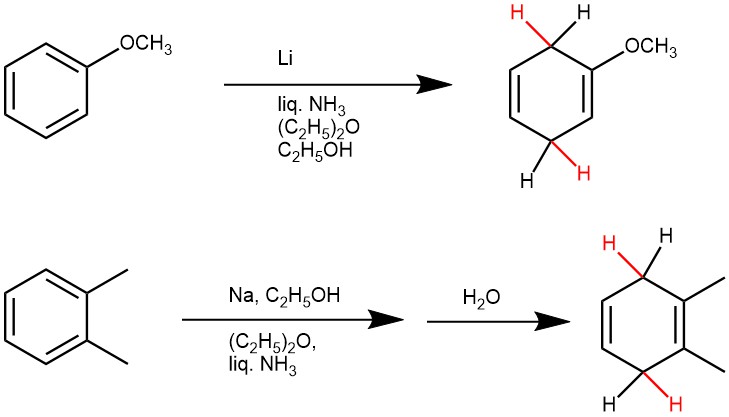
The electron-donating substituents (e.g. -CH3, NH2, -O‾) will reduce the reduction rate, and the substituents are formed on the non-reduced portion of the new product i.e., the product from benzene derivatives with electron-donating groups are usually 1-substituted-2,5-dihydrobenzenes. Also, the bulky substituents on the aromatic rings retard reduction.
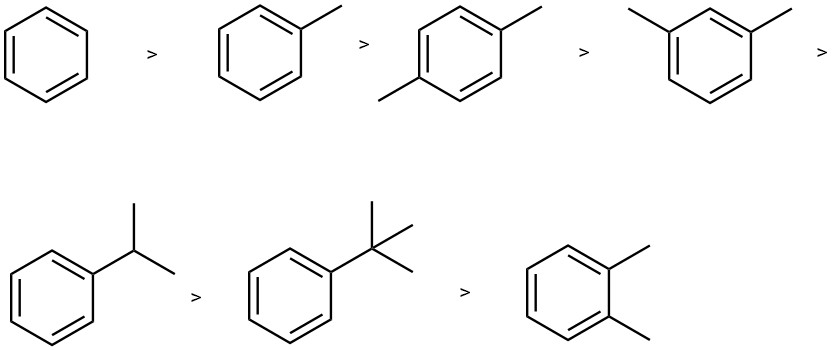
The order of stability for the anion radicals from alkyl-substituted benzene is:
MECHANISM: In the reduction of benzene derivatives, the radical anion is formed reversibly in low concentration and then reacts further with the protonic solvent to form the radical and, subsequently, the anion and the dihydro derivative. An important function of alcohol in the reduction process is to provide a proton source that is more acidic than ammonia.
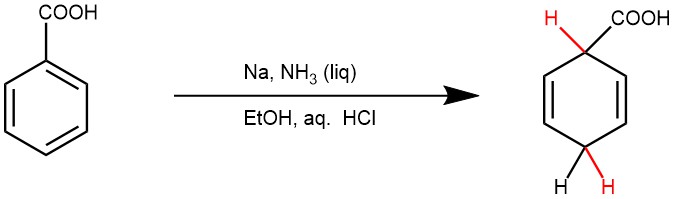
EXAMPLE 1: Ten grams (0.082 mole) of benzoic acid is added to 100 mL of anhydrous ethanol in a 2-L three-necked flask equipped with a mechanical stirrer and with loose cotton plugs in the side necks. After the benzoic acid has dissolved, 600 mL of liquid ammonia is added to the stirred solution. Then 6.2 g (0.27 g atom) of sodium is added in small pieces. When about one-third of the sodium has been added, the white sodium salt of the acid precipitates, and there is strong foaming of the reaction mixture. After all the sodium has been consumed, as evidenced by the disappearance of the blue color, 14.6 g (0.27 mole) of ammonium chloride is added cautiously. The mixture is stirred for an additional hour and then allowed to stand until the ammonia has evaporated.
The residue is dissolved in 300 ml. of water. The solution is poured onto 200 g. of ice and acidified to a pH of about 4 by the addition of 75 mL of 10% hydrochloric acid. The resulting mixture is extracted with four 100-mL portions of peroxide-free ether and the combined extracts are washed with 50 mL of a saturated aqueous solution of sodium chloride and dried over 2 g. of anhydrous magnesium sulfate. The ether solution is separated from the drying agent and concentrated at room temperature under reduced pressure. The residual oil is distilled from a 25-mL Claisen flask with an indented neck.1,4-Dihydrobenzoic acid is obtained as a colorless oil; weight 9.0–9.7 g. (89–95%); b.p. 80–98°/0.01 mm.; n24 1.5011. This material is sufficiently pure for most purposes. However, by a careful redistillation, a small fore-run (b.p. 80–90°/0.01 mm.; n24 1.5000) can be separated, and the remainder of the material (b.p. 91–97°/0.01 mm.; n24 1.5019) solidifies on cooling; m.p. 15–17° (Note 3). It is stored under nitrogen in a closed vessel.[REF: Organic Syntheses, Coll. Vol. 5, p.400 (1973); Vol. 43, p.22 (1963)]
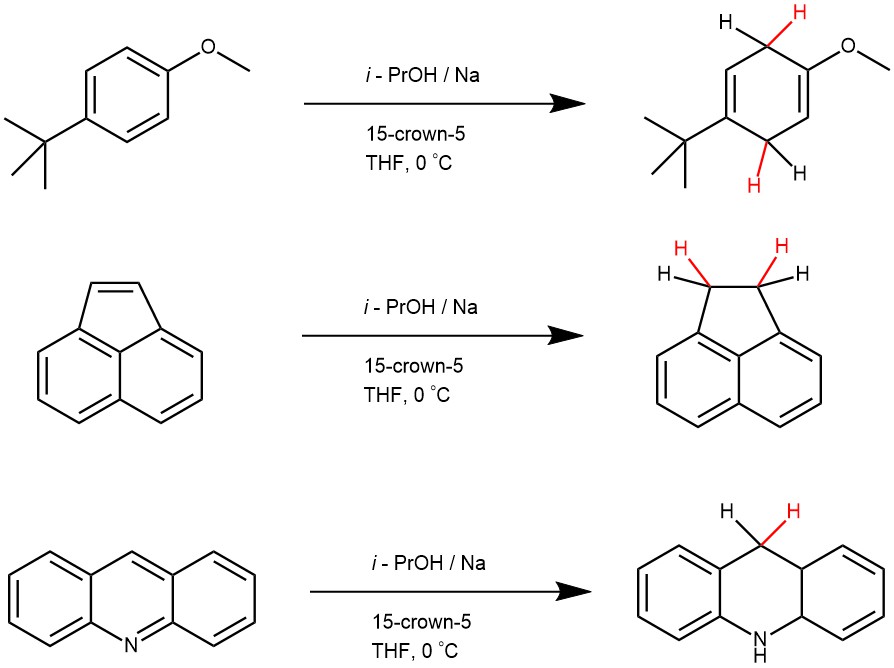
EXAMPLE 2: To a solution of Na dispersion in oil (29.1 wt %, 1.50-4.50 mmol) in anhydrous THF (1.0 mL) was added 15-crown-5 (1.50-4.50 mmol) under N2 at 0 oC and stirred vigorously for 5 min. A solution of substrate (0.500 mmol) and i-PrOH (1.50-4.50 mmol) in THF (2.0 mL) was then added at 0 oC. After the specified time (typically 5-30 min), the reaction was quenched by a saturated aqueous solution of NaHCO3 (2.0 mL), and the reaction mixture was diluted with Et2O (10 mL) and brine (20 mL). The aqueous layer was extracted with Et2O (2 × 10 mL), and the organic layers were combined, dried over MgSO4, filtered, and concentrated. The crude product was purified by flash chromatography (silica, 0-100% EtOAc/hexane).[REF: Org. Lett. 2018, 20, 3439−3442]
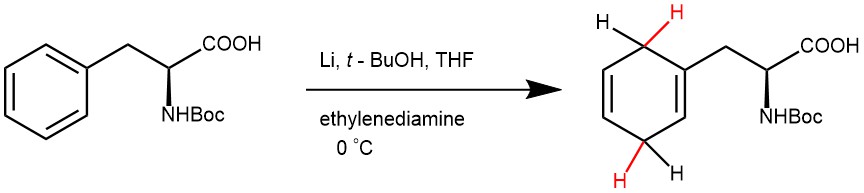
EXAMPLE 3: A single-neck, 100-mL round-bottom flask was equipped with a magnetic stir bar and a septum equipped with a needle connected to a bubbler as a gas outlet. N-(tert-Butoxycarbonyl)-L-phenylalanine (1.00 g, 3.77 mmol), THF (13 mL), ethylenediamine (2.52 mL, 37.7 mmol, 10.0 equiv), and t-BuOH (1.08 mL, 11.3 mmol, 3.0 equiv) were added to the flask, and the resulting solution was stirred while cooling to 0 °C. Lithium (131 mg, 18.9 mmol, 5.0 equiv) was added to the solution. The reaction mixture was stirred at 0 °C (external temperature) until TLC analysis showed the reaction to be complete (30 min).
Saturated aqueous NH4Cl (40 mL) was added to the reaction mixture (CAUTION: Evolution of hydrogen gas), and the resulting mixture was stirred until the remaining lithium was quenched. The resulting mixture was cooled with an ice bath and acidified with o-phosphoric acid until pH = 3–4. The resulting mixture was concentrated under reduced pressure with a rotary evaporator until most THF was removed. The resulting mixture was poured to a 125-mL separatory funnel, and the product was extracted with Et2O (30 mL × 3). The organic extracts were combined, dried over Na2SO4, filtered through cotton, and concentrated under reduced pressure. The resulting residue was purified by flash column chromatography (SiO2, 5 to 50% EtOAc in hexanes with 1% acetic acid) to give (S)-2-((tert-butoxycarbonyl)amino)-3-(1,4-cyclohexadien-1-yl)propanoic acid (551 mg, 54% yield) as a viscous, colorless oil.[REF: Burrows et al., Science 374, 741–746 (2021)]
REFERENCES:
- Modern synthetic reactions by Herbert O. House
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako



