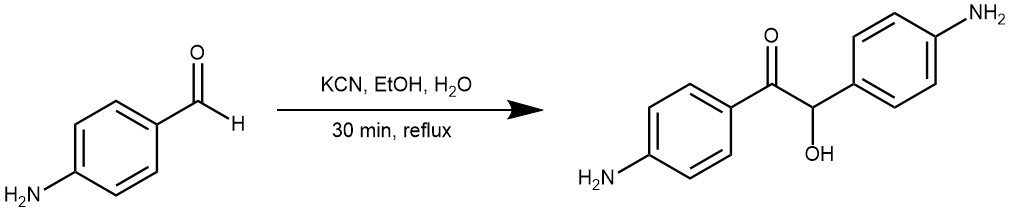
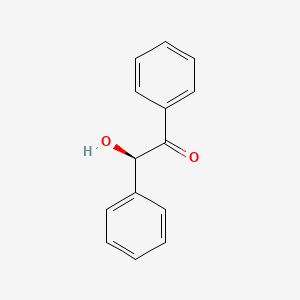
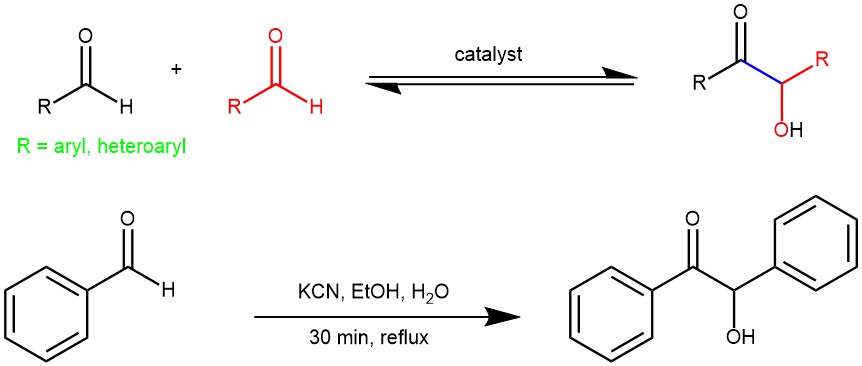
Benzoin condensation is a type of organic reaction that involves the coupling of two aldehydes to form an α-hydroxyketone, also known as an acyloin (Benzoin). The reaction is named after the product obtained when benzaldehyde is used as the starting material, which is benzoin. Benzoin condensation is an important reaction in organic chemistry, as it allows the formation of a new carbon-carbon bond and introduces a hydroxyl group that can be further functionalized. Benzoin condensation was first reported in 1832 by Justus Von Liebig and Friedrich Wöhler, who discovered that benzaldehyde could be converted to benzoin by heating it with potassium cyanide. This condensation involves the addition of one molecule of aldehyde to the C=O group of another aldehyde. One of the aldehyde acts as donor and the other serves as the acceptor.
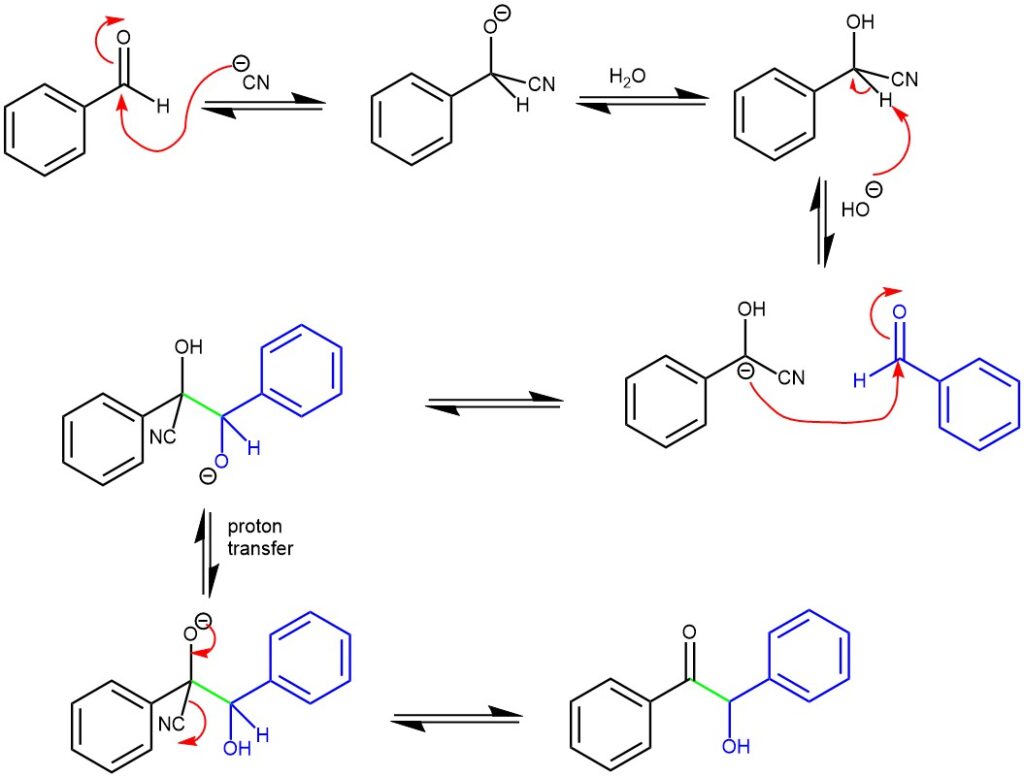
MECHANISM: The reaction mechanism was proposed in 1903 by A. J. Lapworth, who showed that the cyanide ion acts as a nucleophile and adds to the carbonyl group of one aldehyde molecule, creating a cyanohydrin intermediate which is followed by the deprotonation. The deprotonation is possible due to the increased acidity of the C-H bond caused by the electron – withdrawing effect of the CN group. This intermediate undergoes polarity reversal of the carbonyl group, which then adds to another aldehyde molecule in a second nucleophilic addition. Finally, proton transfer and elimination of the cyanide ion yields the benzoin product. Cyanide ion is a very specific catalyst of the reaction as it is a good nucleophile, a good leaving group and its electron-withdrawing effect enhances the acidity of the aldehyde hydrogen.
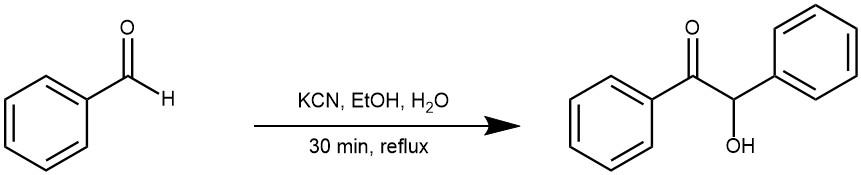
EXAMPLE 1: Place 15 mg of potassium cyanide (poison!) in a reaction tube. Dissolve it in 0.15 ml of water. Add 0.30 mL of 95% ethanol. Add accurately 0.15 mL (157 mg) of pure benzaldehyde. Place a boiling chip in the mixture and reflux the solution on a steam or sand bath for 30 minutes. On completion of reflux, cool the tube in an ice bath. Crystals of benzoin appear on cooling. Induce crystallization by withdrawing a drop on the stirring rod and rub it against the side of the tube. Remove the solvent with a Pasteur pipette and wash the crystals with 1 mL of 1:1 mixture of 95% ethanol and water. Filter the colourless crystals of benzoin on a Hirsh funnel.[ J. Chem. Educ. 2020, 97, 5, 1336–1344]
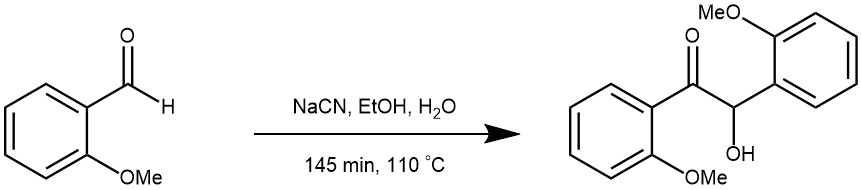
EXAMPLE 2: To a solution of 2-methoxyhenzaldebyde (16 g, 117 mmol) in 95% ethanol (17 ml) was added a solution of NaCN (2.45 g, 1.2 mmnol) in water (13 ml) and the mixture was heated at 110 °C with stirring for 45 min followed by heating on a steam-bath for 30 min. The reaction mixture was decomposed by crushed ice and allowed to stand overnight, whence 12 g (75%) of product was obtained, crystallised further from dichloromethane-petroleum ether, 60-80 °C mixture to yield pure colorless crystals. [REF: Tetrahedron 55 (1999) 11537-11546]
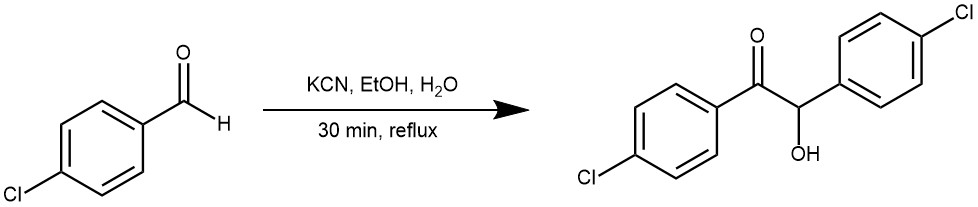
EXAMPLE 3: To a solution of 4-chloro-benzaldehyde (0.57 g, 2.04 mmol) in EtOH (8 mL) is added KCN (18.0 mg, 0.27 mmol) dissolved in water (4 mL). The mixture is heated to reflux for 7 hours, then cooled to room temperature and extracted with ethyl acetate (100 mL). The organic layer is dried (MgSO ), filtered, and concentrated. The residue is purified by flash chromatography with 40% ether/hexane to give a solid. [REF: World Intellectual Property Organization WO2005116000]
EXAMPLE 4: Dissolve about 4 g of KCN in 75 mL of water in a one-litre flask. Add 14 g of 4-amino benzaldehyde and 75 mL of 95% ethanol into the flask. Reflux the mixture formed into a solution at the boiling temperature for one and half hour. Pass steam through the solution until remove all the alcohol and nearly all the unreacted aldehydes. Decant the condensed water from the product and set latter aside for crystallisation. Press the product as free as possible from oily material on a suction funnel and wash with cold alcohol. [REF: Oxidation Communications (2021), 44(4), 703-714]