The process of transforming benzil, an α-diketone, into the salt of α-hydroxy acid, followed by the conversion to benzylic acid upon acidification, is commonly known as the benzilic acid rearrangement or benzil-benzilic acid rearrangement. This rearrangement is typically performed using water, aqueous ethanol, or other aqueous organic solvents like aqueous dioxane, and it can even be carried out in a solid state. Under refluxing conditions, this rearrangement has been observed to reach completion within a few hours.
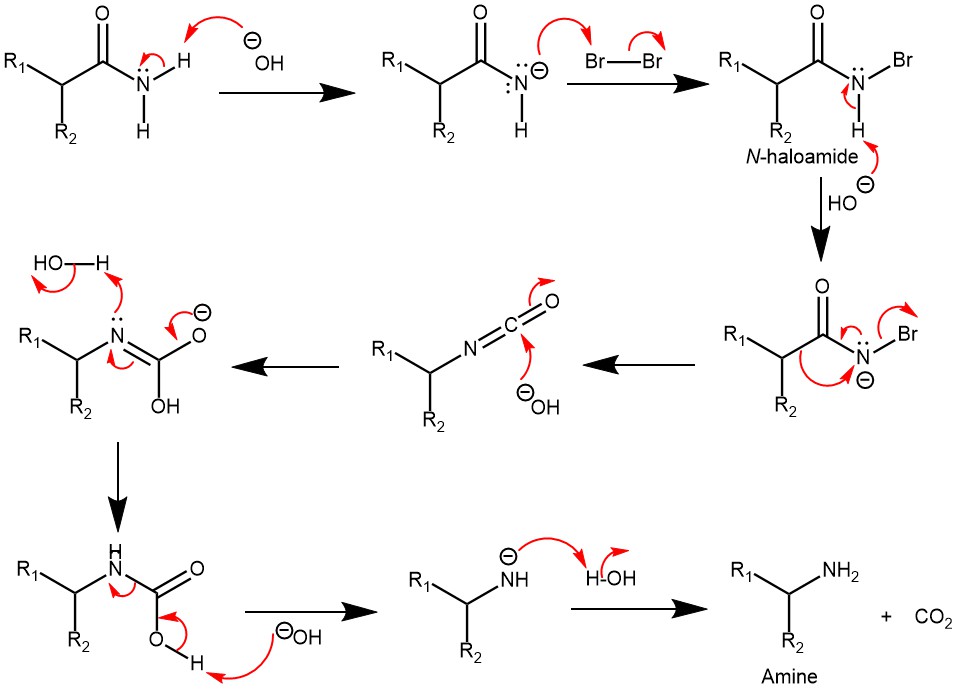
The benzylic acid rearrangement is formally the 1,2-rearrangement of 1,2-diketones to form α-hydroxycarboxylic acid using a base. The reaction receives its name from the reaction of benzil with potassium hydroxide to form benzylic acid. The reaction was first performed by Justus Leibig in 1838. The Aryl group tends to migrate more rapidly than the alkyl group. When two different aryl groups are available, the major product usually results from the migration of the aromatic ring with the more powerful electron-withdrawing group.
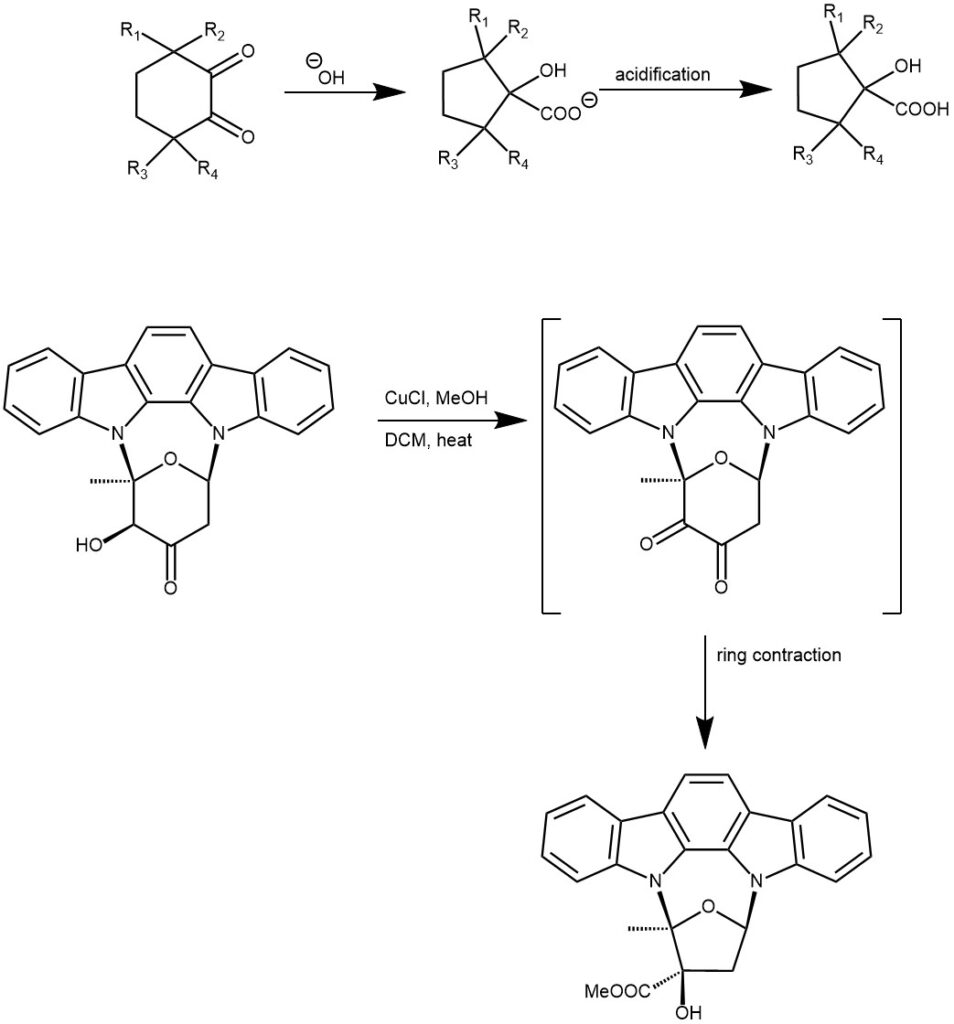
The reaction takes place with both aliphatic and aromatic α-keto aldehydes. Cyclic α-diketones undergo the ring-contraction benzylic acid rearrangement under these conditions.
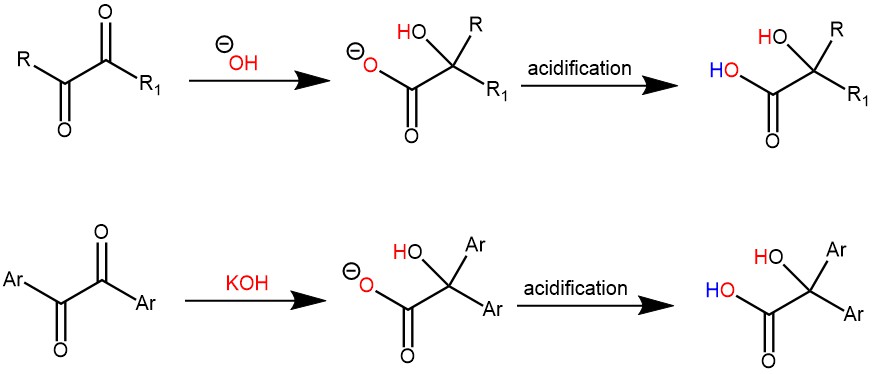
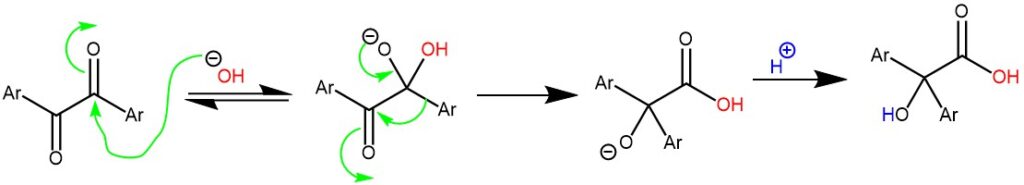
MECHANISM: The initial step of the mechanism involves the nucleophile (such as hydroxide, alkoxide, or amide ion) adding to the carbonyl carbon, resulting in the formation of a tetrahedral intermediate. Subsequently, an aryl or alkyl group migrates within the molecule, leading to the formation of the corresponding salt of α-hydroxy acid. Upon acidification, this salt is converted into the α-hydroxy acid.
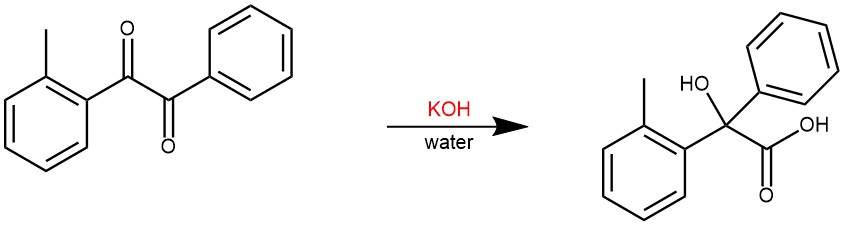
EXAMPLE 1: A solution containing 130 mg. of 2-methyl benzil, 550 mg. of potassium hydroxide, 1 ml. of alcohol, and 1.5 ml. of water was heated for 10 min. on the steam cone. The solution was cooled, diluted with water, and extracted with ether. There was recovered 29 mg. of 2-methyl benzil from this ether extraction of the reaction mixture. The aqueous layer was acidified and extracted with ether. This ether extract was dried and evaporated and the residue was recrystallized to give 105 mg. (96% based on unrecovered starting material) of 2-methyl-benzilic acid C14, m.p. 113.5-114.5° (lit.· 114.5°), 1.443 ±0.001 mc./mole. [REF: J. Org. Chem. 1958, 23, 11, 1764–1766]
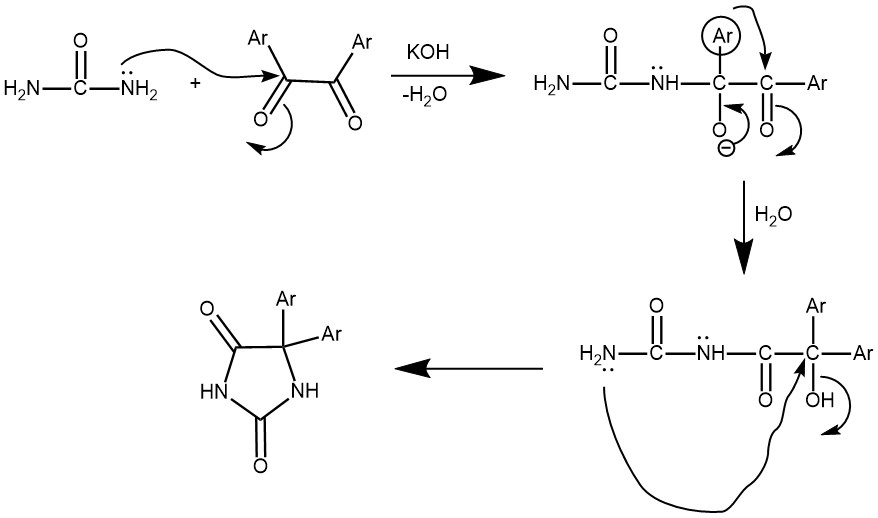
EXAMPLE 2: Dissolve 10.1 g (0.05 mol) of benzil, and 6.0 g (0.1 mol) of urea in 100 ml of ethanol in a 250-ml round-bottom flask, and add to this a solution of 16.5 g (0.3 moi) of potassium hydroxide in 20 ml of water. After refluxing the mixture for 2.5 h, the flask is cooled and the solids are filtered off. The alkaline filtrate is cooled in an ice-water bath and slowly acidified with 6N sulfuric acid to pH 3, during which the hydantoin precipitates out of the solution. The product is filtered and air dried to yield 5,5′-diphenylhydantoin (phenytoin), mp 295-298°C with decomposition.3 Recrystallization can be accomplished from 95% ethanol, although the product isolated by acidification of the filtered reaction mixture is usually better than 95% pure.[REF: Journal of Chemical Education: The synthesis of 5,5’- diphenylhydantoin: A novel benzil-benzilic acid rearrangement]
REFERENCES:
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako