In the field of medicinal/organic chemistry, the amide coupling reaction has become very popular in recent years. The amide bond is a fundamental component of proteins and is formed by condensing a carboxylic acid and an amine. This reaction is preferred by chemists because there is a vast range of carboxylic acid and amine derivatives that are easily accessible, making amide coupling an efficient method for creating new compounds. Therefore, a wide variety of reagents and protocols have been developed to enable this straightforward amide bond formation.
The most common method for the formation of an amide bond is the condensation of a carboxylic acid and an amine. However, this direct condensation reaction is very slow in most cases and this direct coupling between a carboxylic acid and an amine is hardly a suitable choice in synthetic chemistry because of the competing acid/base proton exchange.
The most common strategy is the conversion of the acid to an activated form (i.e. more electrophilic), such as acyl chloride or anhydride. These species readily react with primary and secondary amines to give the corresponding amide. Most commonly, the reaction proceeds rapidly at room temperature in aprotic solvents in the presence of a suitable base, such as a tertiary amine or pyridine. Acylation of amine by acyl chloride is often referred to as Schotten–Baumann reaction, from the names of its inventors. The Schotten-Baumann reaction and the coupling between the amine and an anhydride are mechanistically related, the only significant difference being the acid by-product: HCl in one case, a carboxylic acid in the other. They both require a base to drive the equilibrium to the right.

HATU
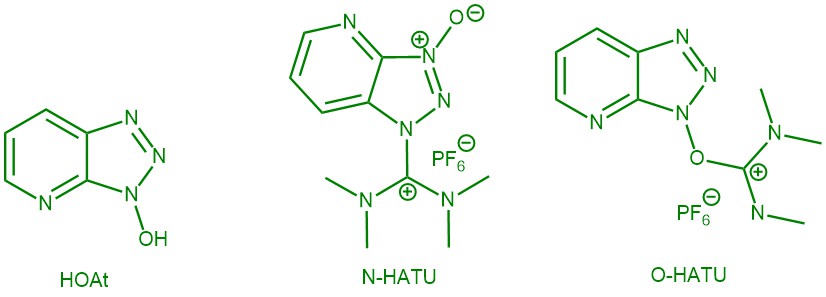
[N-[(dimethylamino)-1 H-1,2,3-triazolo[4,5- b]pyridin-1-ylmethylene]-N-methylmethanaminium hexafluorophosphate N-oxide ] [HATU]: Significant progress in the synthesis of amides has been achieved in the last three decades. The big variety of amide coupling reagents that are commercially available nowadays shares a fundamental chemical principle: the synthesis of a highly activated ester. HATU is one of the very promising peptide coupling reagents to generate an active ester from a carboxylic acid. It was first reported by Louis A. Carpino in 1993 as an efficient means of preparing active esters derived from 1-hydroxy-7-azabenzotriazole (HOAt). HATU can exist as either the uronium salt (O-form) or the less reactive iminium salt (N-form). Originally HATU was classified as O-uronium salt (O-HATU) but later revised as N-guanidinium salt (or aminium salt) (N-HATU). O-HATU is more efficient coupling reagent than the corresponding N-HATU.
MECHANISM OF N-ACYLATION USING O-HATU:
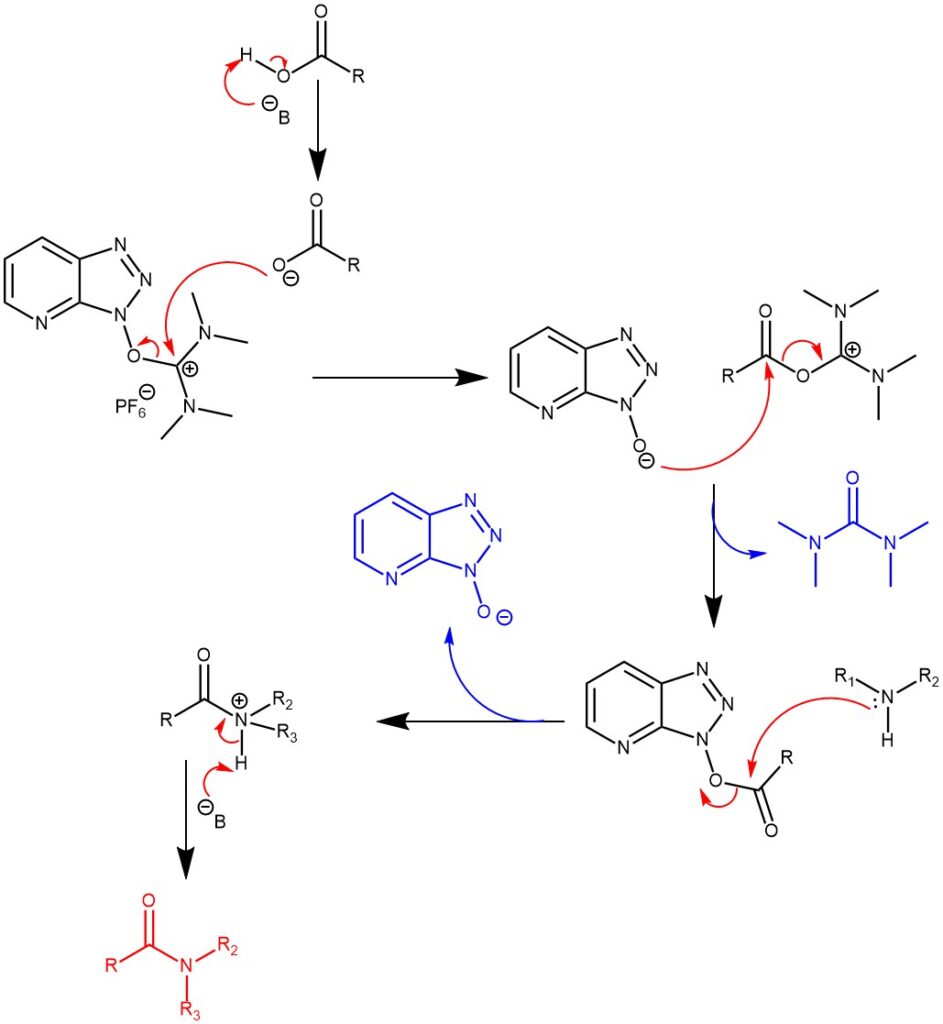
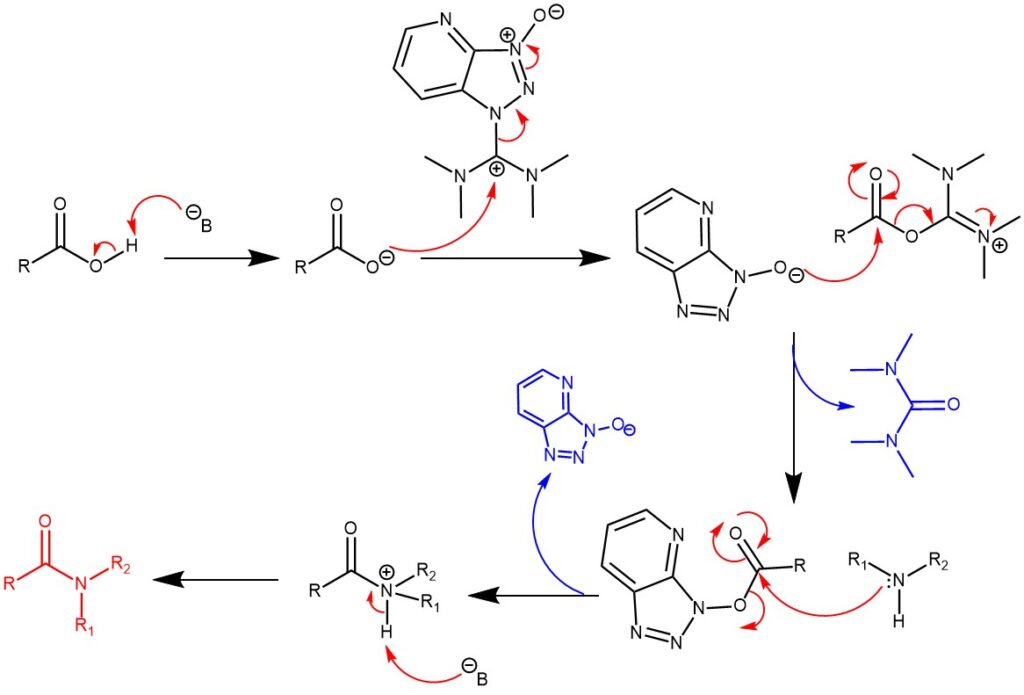
MECHANISM OF N-ACYLATION USING N-HATU:
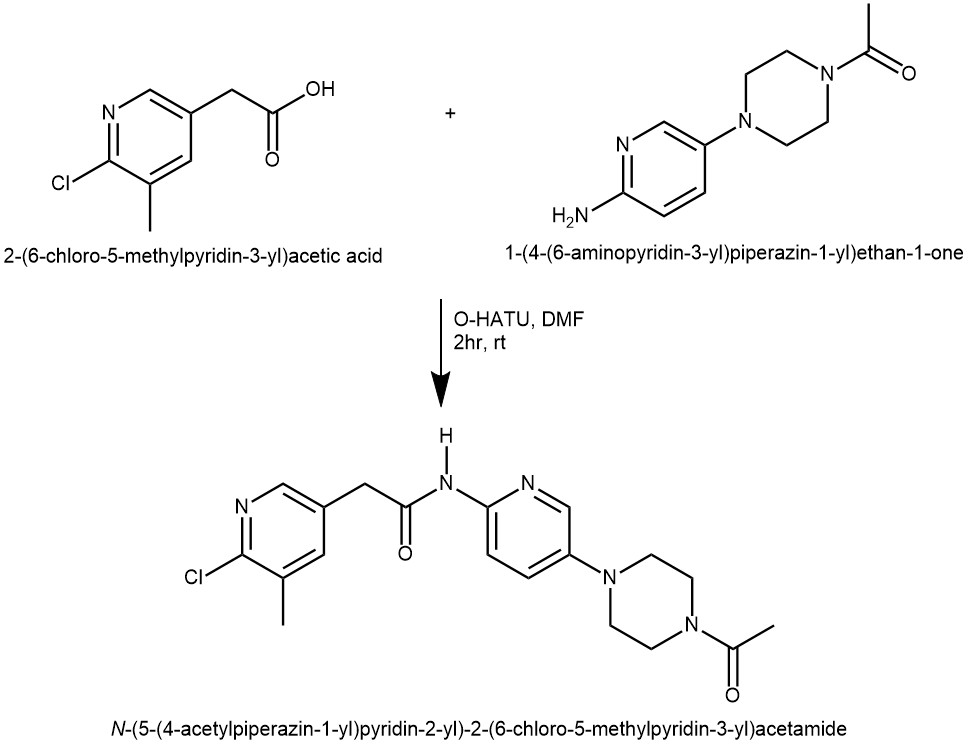
EXAMPLE 1: To a mixture of 2-(6-chloro-5-methylpyridin-3-yl)acetic acid 74-4 (100 mg, 0.54 mmol),1-(4-(6-aminopyridin-3-yl)piperazin-1-yl)ethanone 111-4 (140 mg, 0.64 mmol) and O-(7-azabenzotriazol-1-yl)-N,N,N’,N’-tetramethyluroniumhexaflurophosphate (HATU) (220 mg, 0.58 mmol) in DMF (2 mL) was added DIEA (280 μL, 1.62 mmol) at room temperature. The mixture was stirred at room temperature for 2 hours. The reaction mixture was diluted into ethyl acetate, washed with saturated NaHCO3 then brine, dried over Na2SO4. The solvent was removed by rotary evaporation to give N-(5-(4-acetylpiperazin-1-yl)pyridin-2-yl)-2- (6-chloro-5-methylpyridin-3-yl)acetamide 148-1 (210 mg, 100%). MS m/z 388.1 (M + 1). (ref: World Intellectual Property Organization, WO2010101849 A1 2010-09-10)
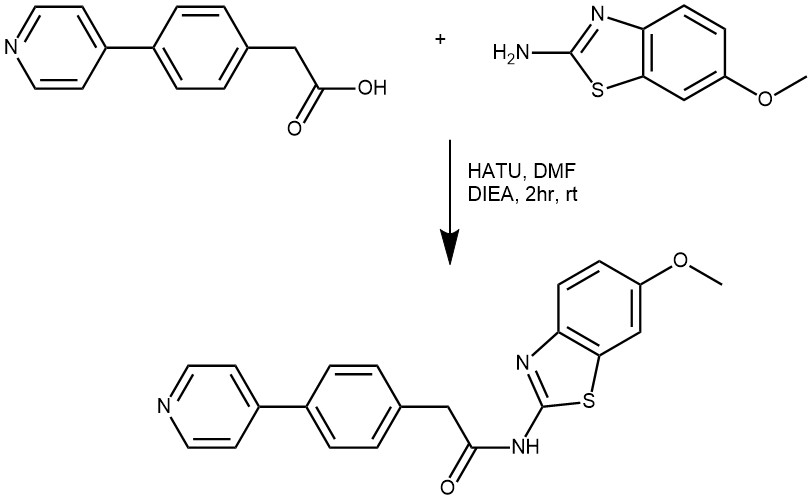
EXAMPLE 2: To a mixture of 2-(4-(pyridin-4-yl)phenyl)acetic acid (45 mg, 0.21 mmol), 6 -methoxybenzo[d]thiazol-2-amine (36 mg, 0.20 mmol), and DIEA (32 mg, 0.25 mmol) inDMF (0.5 mL) under stirring was added HATU (84 mg, 0.22 mmol). The solution was stirred for2 hours before it was subject to reverse phase HPLC for purification to give the product as whitesolid. MS m/z 376.1 (M + I). (Ref: World Intellectual Property Organization WO 2010/101849 Al)
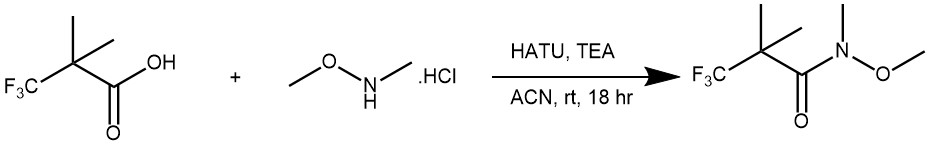
EXAMPLE 3: o a solution of the acid (5.00 g, 32.0 mmol) in ACN (22.9 mL) was added TEA (9.82 mL, 70.5 mmol) followed by HATU (12.8 g, 33.6 mmol). The mixture was stirred at RT for 15 min, after which time the dark clear mixture was treated with the amine (3.44 g, 35.2 mmol). The reaction mixture was stirred at RT for 18 h. The mixture was diluted with EtOAc (100 mL) and washed with 1N HCl (2 x 100 mL), sat. NaCl (5 x 100 mL), dried (Na2SO4), and concentrated. The resulting crude orange solid was adsorbed onto a plug of silica gel and purified by chromatography (0-25% EtOAc/heptane) to provide the product as a yellow liquid. [5.050 g, 79%] (Ref: World Intellectual Property Organization WO2015129926)
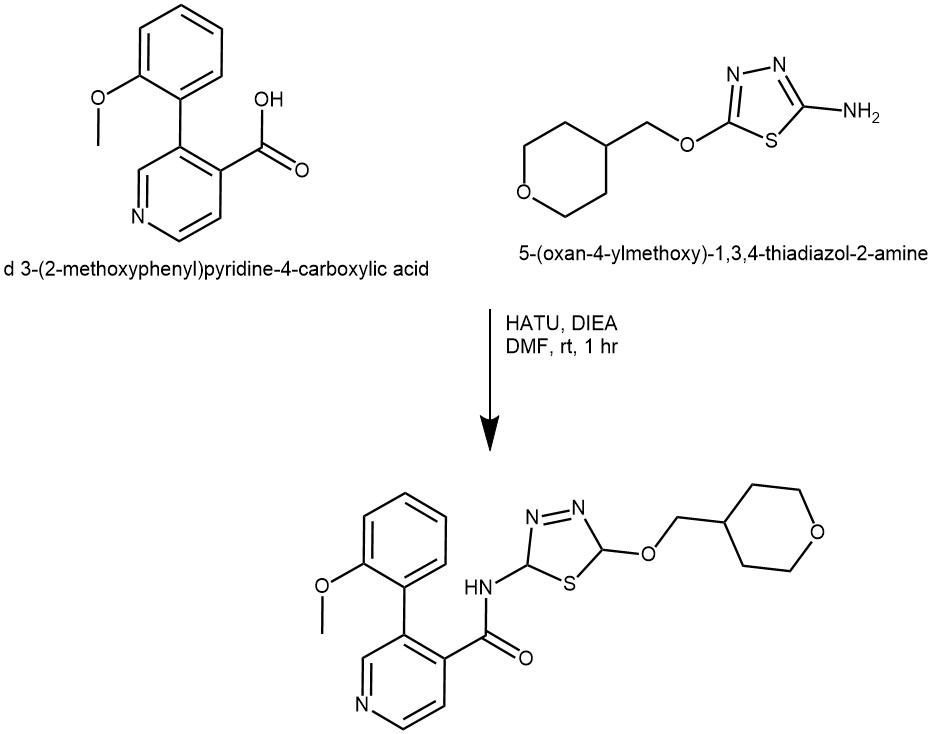
EXAMPLE 4: To a stirred solution of 5-(oxan-4-ylmethoxy)-l,3,4-thiadiazol-2-amine (34.00 mg, 0.158 mmol, 1.00 equiv) and 3-(2-methoxyphenyl)pyridine-4-carboxylic acid (36.21 mg, 0.158 mmol, 1.00 equiv) in DMF (400.00 uL) was added DIEA (40.83 mg, 0.316 mmol, 2.00 equiv) and HATU (72.06 mg, 0.190 mmol, 1.20 equiv) at room temperature . The resulting mixture was stirred for 1 hour at rt. The reaction was diluted with EA (20 mL). The resulting mixture was washed with 2×10 mL of water. The organic layer was concentrated under a vacuum. The residue was purified by Prep-TLC (CH2CI2 / MeOH 15:1) to afford 3-(2-methoxyphenyl)-N-(5-((tetrahydro-2Hpyran-4-yl)methoxy)-l,3,4-thiadiazol-2-yl)isonicotinamide (68.2mg, 101.15%) as a white solid. (Ref: World Intellectual Property Organization WO 2022/118210 Al)