Diazonium salt is a versatile and important class of compounds in organic chemistry. It has played a significant role in the development of the chemical industry, particularly in the production of dyes and pharmaceuticals. The discovery of diazonium salt is attributed to the German chemist Peter Griess, who first synthesized it in 1858. Since then, the compound has been extensively studied and used in a wide range of chemical reactions and applications.
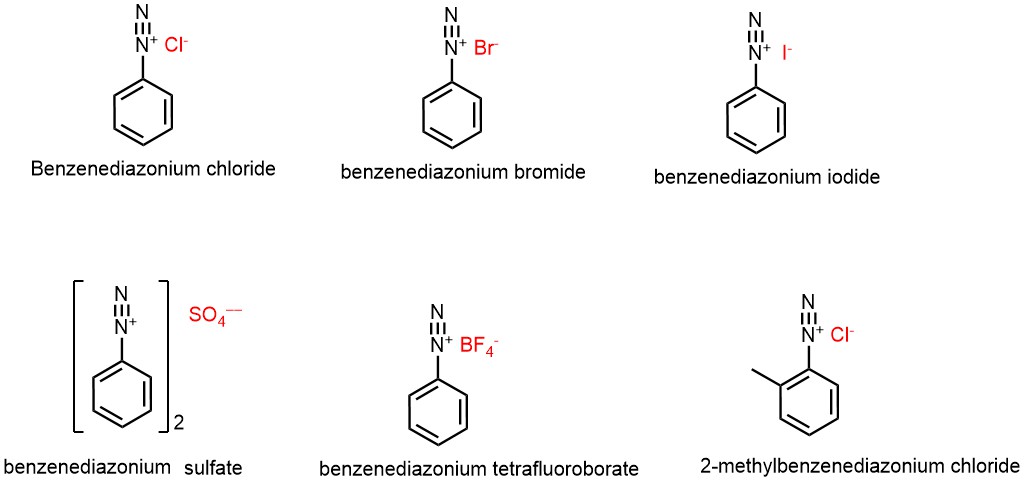
Diazonium salt is a type of organic salt that contains a positively charged nitrogen atom (N2+) and a negative counterion. There are several possible negative counterions that can be associated with diazonium salts, depending on the method of preparation and the specific application. Some of the most commonly used counterions include:
- 1. Chloride ion (Cl‾)
- 2. Bromide ion (Br‾)
- 3. Iodide ion (I‾)
- 4. Tetrafluoroborate ion (BF4‾)
- 5. Hexafluorophosphate ion (PF6‾)
- 6. Sulfate ion (SO4 ‾ ‾)
- 7. Nitrate ion (NO3‾)
- 8. Acetate ion (CH3COO‾)
Diazonium compounds are ionic in nature i.e., they are salts. This is well reflected in their name as they are collectively known as “Diazonium salts” (the word “di” refers to “two”, “aza” stands for “nitrogen” and the last term “onium” suggests the ionic nature of the compound). Thus ionic compounds containing N≡N are collectively called diazonium salts and they are named by appending the word “Diazonium” to the name of the aromatic compound, followed by the name of the anion.
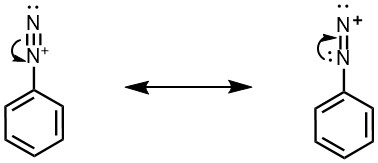
Benzenediazonium ion can thus be represented by following two electronic structures, in which the positive charged is shared by both the nitrogen atoms.
Diazonium salts can be synthesized by the reaction of primary aromatic amines with nitrous acid (HNO2) in the presence of an acid. The general reaction can be represented as follows:
Ar-NH2 + HNO2 + HX → Ar-N2X- + 2H2O
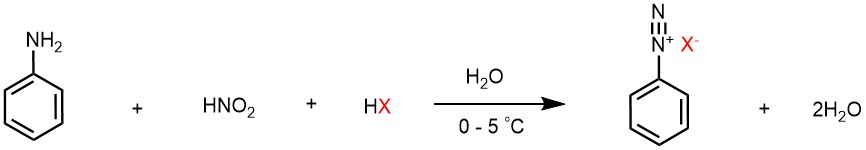
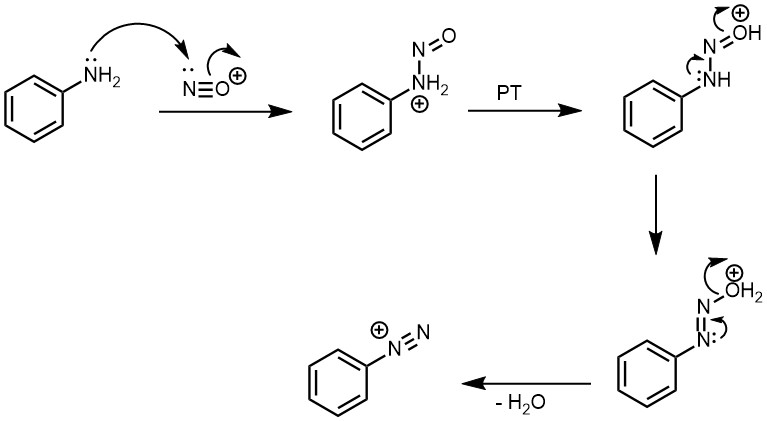
In this reaction, Ar represents an aromatic group (such as phenyl or naphthyl), X represents a counterion (such as Cl-, Br-, or BF4-), and HX represents an acid (such as hydrochloric acid or sulfuric acid) that helps to protonate the amine and stabilize the diazonium intermediate. But since nitrous acid is unstable, it is generated in situ by treatment of sodium nitrite with strong acid such as HCl or H2SO4. This process of conversion of primary amine to its diazonium salt is known as diazotization. The diazonium salts of primary aromatic amines are also unstable, but they are far more stable in comparison to diazonium salts of aliphatic primary amines, and they do not decompose when the temperature of the reaction mixture is kept below 5 °C.
Aryl diazonium salts are commonly prepared by treatment of the corresponding aryl amine (like aniline) with a nitrite (like sodium nitrite) and an acid (like hydrochloric acid) in an aqueous medium. For example, benzene diazonium chloride can be prepared by reacting aniline with sodium nitrite and hydrochloric acid in aqueous medium.
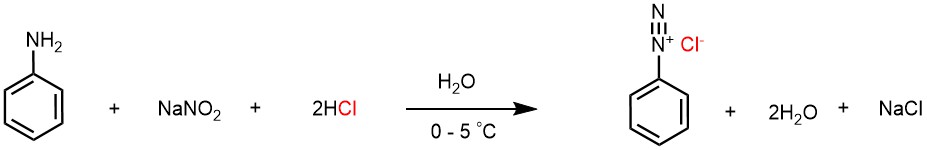
Since nitrous acid is unstable, it has to be prepared in situ by the action of hydrochloric acid over sodium nitrite. The hydrochloric acid protonates the sodium nitrite to form the nitrous acid.
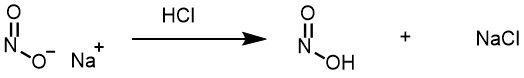
The hydrochloric acid serves further purpose as well; it protonates the -OH of nitrous acid, followed by the loss of water, produces a strong electrophile called as nitrosonium ion (NO+)
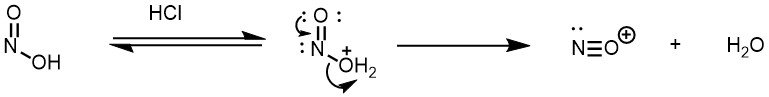
The aromatic amine then attacks the nitrosonium ion followed by the proton transfers from the nitrogen of amine to the oxygen (tautomerization) and finally the loss of water gives the diazonium compounds.
The nucleophilicity of the amino nitrogen in the diazotization reaction depends on the presence of electron-withdrawing or electron-donating groups on the aromatic ring. Electron-withdrawing groups (-M and -I) decrease the electron density on the nitrogen atom, reducing its nucleophilicity and making it harder for the diazotization reaction to occur. p-nitroaniline is an example of an electron-withdrawing group, making it a weaker base compared to aniline.
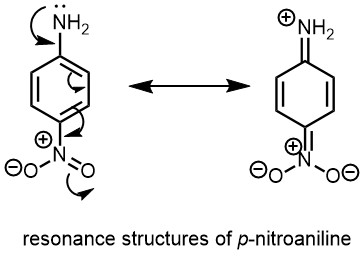
On the other hand, electron-donating groups (+M and +I) increase the electron density on the nitrogen atom, increasing its nucleophilicity and accelerating the diazotization reaction. Groups such as -OH, -NH2, -OCH3, and -NHR all have electron-donating effects and can increase the rate of the diazotization process when present at the ortho or para positions to the amino group.
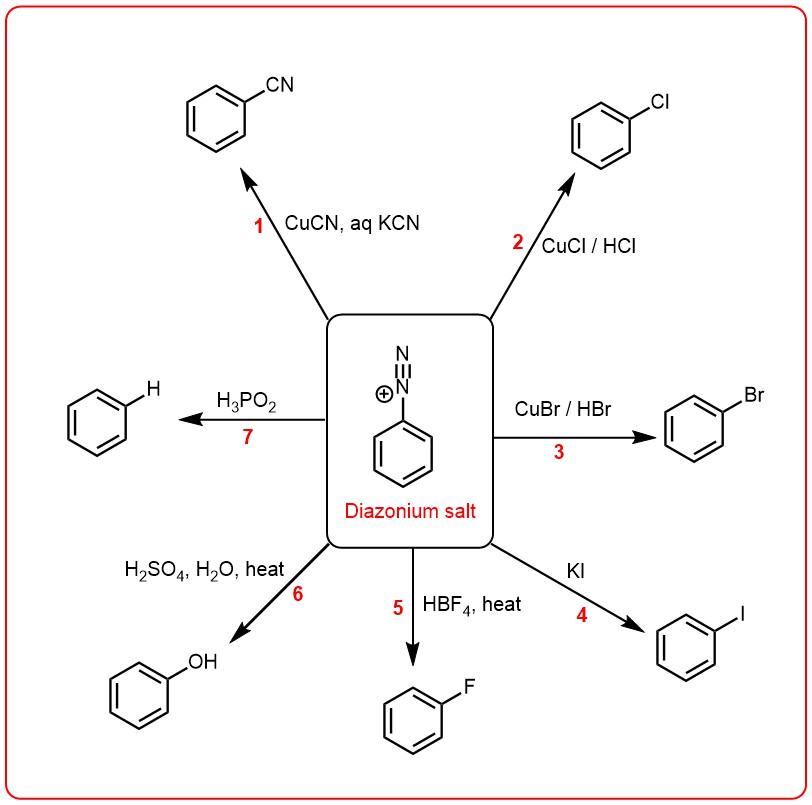
REACTIONS OF DIAZONIUM SALTS:
Diazonium salts have a versatile chemistry and can undergo a range of reactions that depend on the nature of the diazonium salt and the reaction conditions. These reactions are classified into two main groups based on whether the diazo group is retained or replaced in the final product.
In the first group, the diazo group is replaced by another functional group, resulting in the formation of a new compound. This group of reactions includes Sandmeyer reaction, Gattermann reaction, and Schiemann reaction, among others. These reactions are used for the synthesis of a range of compounds, including aryl halides, aryl nitriles, and aryl sulfonates.
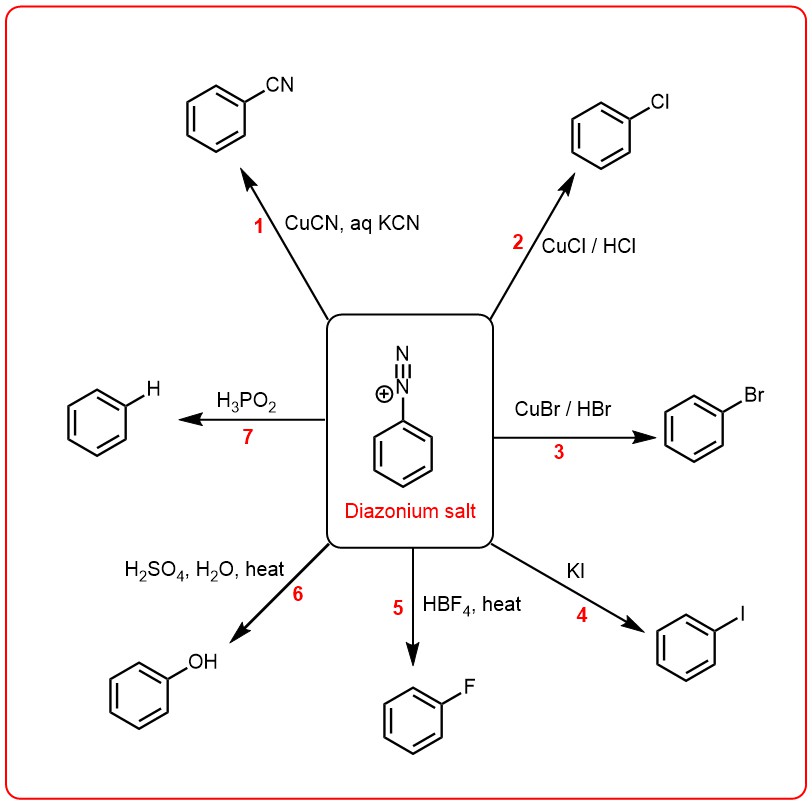
SANDMEYER REACTIONS:
Diazotization of primary amines with nitrous acid, followed by treatment of the diazonium salt with cuprous chloride (CuCl), cuprous bromide (CuBr) yields the corresponding aryl chloride and aryl bromide respectively. Reactions 2 and 3 above are classified as Sandmeyer reactions. The replacement of diazo group by cyano group (1) is a special case of Sandmeyer reaction. This involves treatment of the diazonium salt solution with a solution of copper (I) cyanide in aqueous potassium cyanide.
GATTERMANN REACTION:
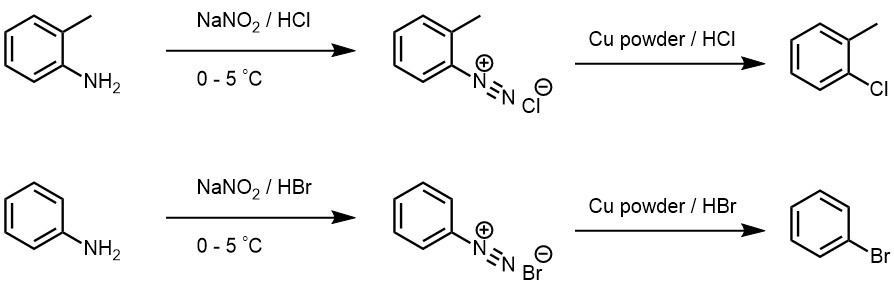
This reaction named after German chemist Ludwig Gatterman is originally used for the synthesis of aromatic aldeydes, but synthesis of aryl halides from diazonium salt are also included into this reaction. The reaction is a modification of Sandmeyer’s reaction and instead of cuprous salts, finely divided copper is used for the decomposition of diazonium salt solution.
COUPLING REACTIONS:
In the second group, the diazo group is retained in the final product, resulting in the formation of a compound that contains the diazo group. This group of reactions includes the coupling reaction, which is used for the synthesis of azo compounds, and the reduction reaction, which is used to convert the diazonium salt into a primary aromatic amine. Coupling reactions of diazonium salts are chemical reactions in which a diazonium ion is reacted with an aromatic or heteroaromatic compound that contains an activated position (such as a phenol, aniline, or an activated benzene ring) to form an azo compound. This reaction is known as an azo coupling reaction. The reaction involves the nucleophilic attack of the activated aromatic compound on the diazonium ion, followed by the formation of an unstable intermediate, which then rearranges to form the azo compound. The reaction proceeds under basic or acidic conditions, depending on the nature of the reactants and the desired product.
The general equation for the azo coupling reaction is:
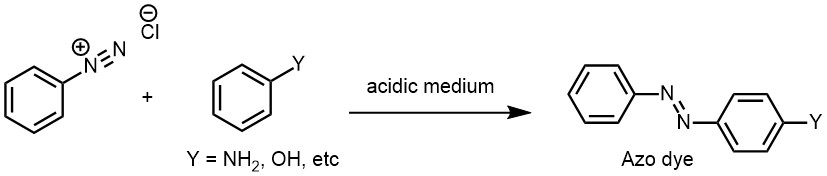
Azo coupling reactions are important in the synthesis of dyes, pigments, and other organic compounds. The resulting azo compounds exhibit a range of colors depending on the nature of the aromatic groups and the substitution pattern on the aromatic ring. The color of the azo compound is due to the presence of a chromophore, which is a group of atoms that absorbs light at specific wavelengths and gives the compound its color. Diazonium salts are weak electrophiles, and they tend to react with those aromatic systems that are activated by strong electron-donating groups. This is because electron-donating groups increase the electron density on the aromatic ring, making it more nucleophilic and more reactive towards electrophilic diazonium ions. Diazonium salts can undergo coupling reactions with phenols and aromatic amines in alkaline, neutral, or mild acidic medium to yield highly colored azo compounds. This type of coupling is often referred to as C-coupling because it involves the coupling of two carbon atoms in the aromatic ring.
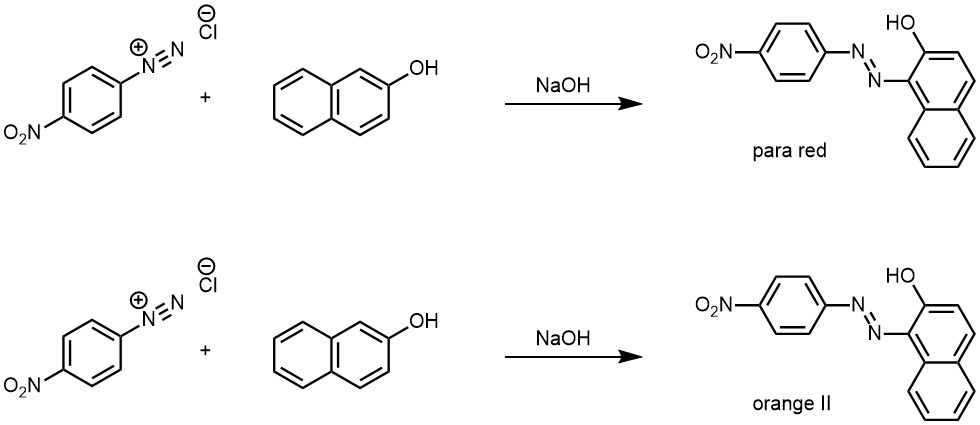
When diazonium salts are coupled with phenols, the coupling reaction is usually carried out in the pH range of 8-11, where phenols are present as phenoxide ions. The presence of phenoxide ions makes the phenols more reactive towards electrophilic diazonium ions, leading to the formation of highly colored azo compounds.
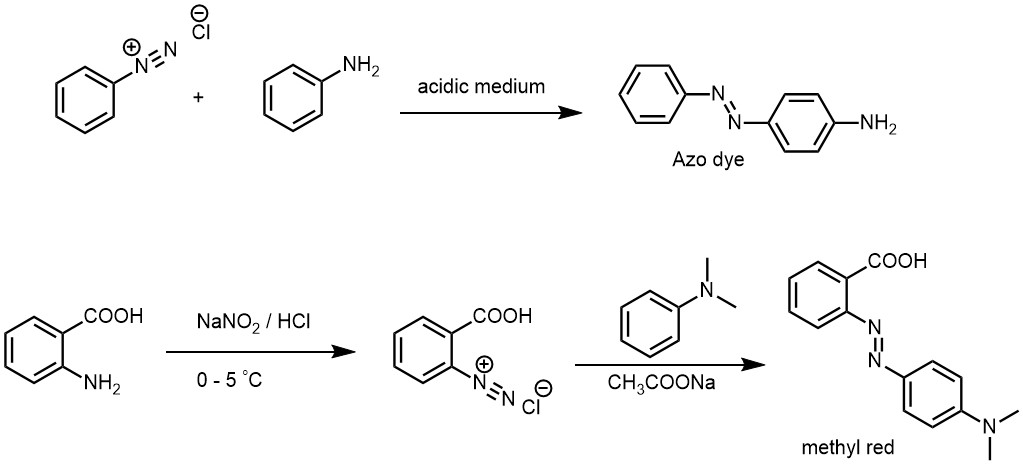
Similarly, when diazonium salts are coupled with amines, the coupling reaction is carried out in slightly acidic medium. Tertiary amines react with diazonium salts in a similar manner as phenols, where the presence of the amine group activates the aromatic ring towards electrophilic attack by the diazonium ion.
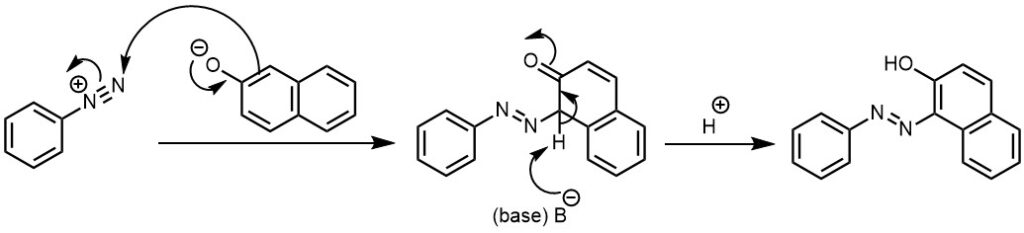
MECHANISM:
There are many important azo dyes that can be prepared by azo coupling reactions. Here are a few examples:
Methyl orange: This is a pH indicator dye that is used to determine the acidity or basicity of a solution. It is prepared by coupling sulfanilic acid with N,N-dimethylaniline.
Congo red: This is a pH indicator dye that is used to distinguish between acidic and basic dyes. It is prepared by coupling benzidine with sulfonated naphthalene.
Orange II: This is a dye that is used in the food industry to color cheese and other products. It is prepared by coupling 2-naphthol with N,N-dimethylaniline.
Direct Yellow 4: This is a yellow dye that is used in the textile industry to dye cotton and other fibers. It is prepared by coupling diazotized p-nitroaniline with N,N-dimethylaniline.