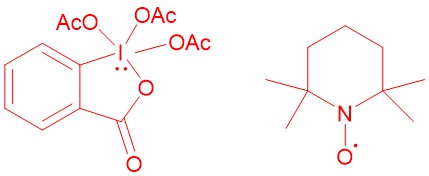
DESS-MARTIN PERIODINANE (DMP):
In 1983, D. B. Dess and J. C. Martin reported DMP (Dess-Martin Periodinane). This periodinane, which is far more soluble in organic solvents than IBX (2-Iodoxybenzoic acid), is a mild, selective reagent for the oxidation of primary and secondary alcohols to aldehydes and ketones respectively.
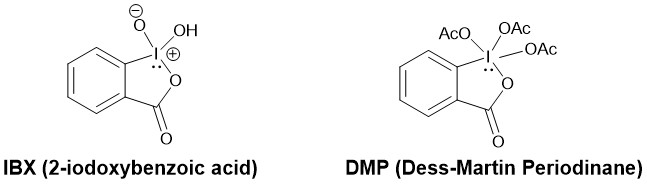
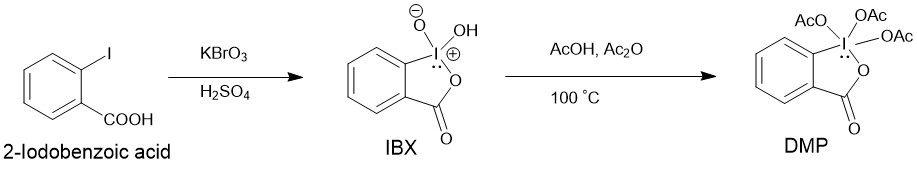
Since DMP is expensive, it is usually prepared by oxidation of 2-iodobenzoic acid to IBX with potassium bromate (KBrO3) in aqueous sulfuric acid followed by acylation of IBX to DMP using acetic acid and acetic anhydride.
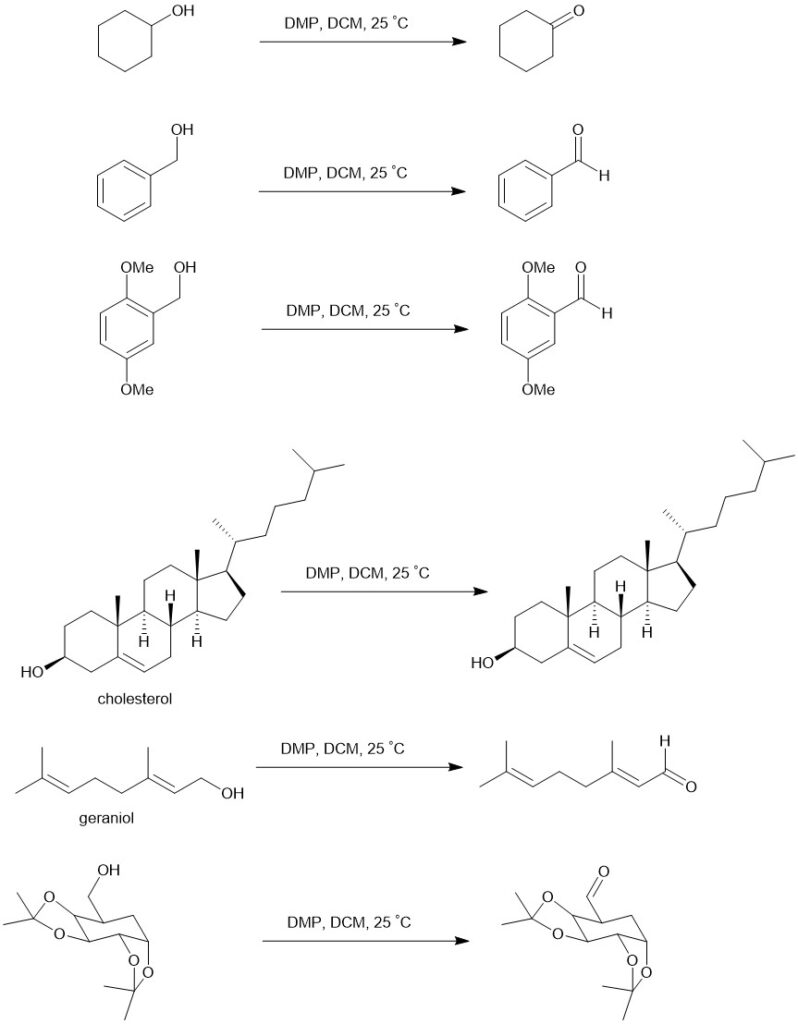
It is sparingly soluble in ether and hexane but highly soluble in other organic solvents like chloroform, dichloromethane (DCM), and acetonitrile. In a typical experiment, a solution of the alcohol in DCM is added to a solution of DMP in DCM with stirring. One equivalent of DMP can oxidize one equivalent or less of a primary or secondary alcohol at room temperature to the respective aldehyde or ketone without further oxidation to carboxylic acid. It reacts more rapidly with benzylic alcohols than with saturated alkanols.
Eg.
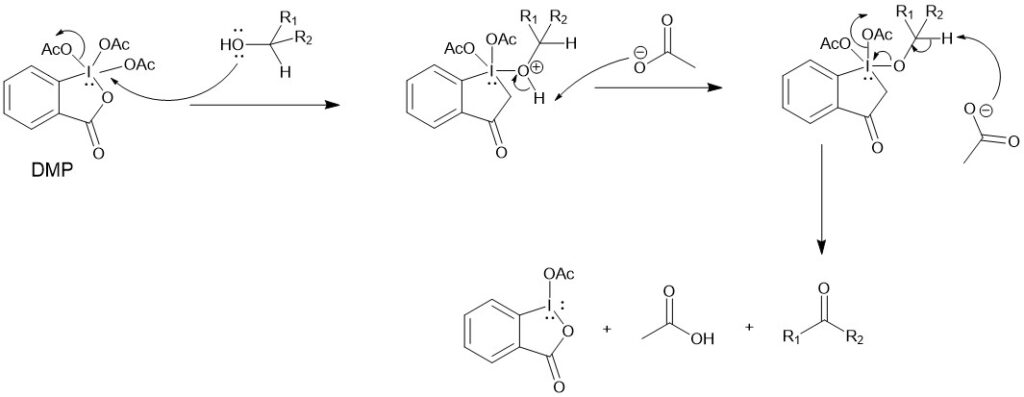
MECHANISM:
DMP reacts rapidly with alcohol to give diacetoxyalkoxy periodinane. The α-proton of the alcohol is then removed by a base (acetate ion) to give the carbonyl compound.
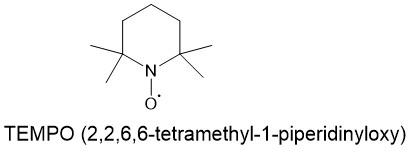
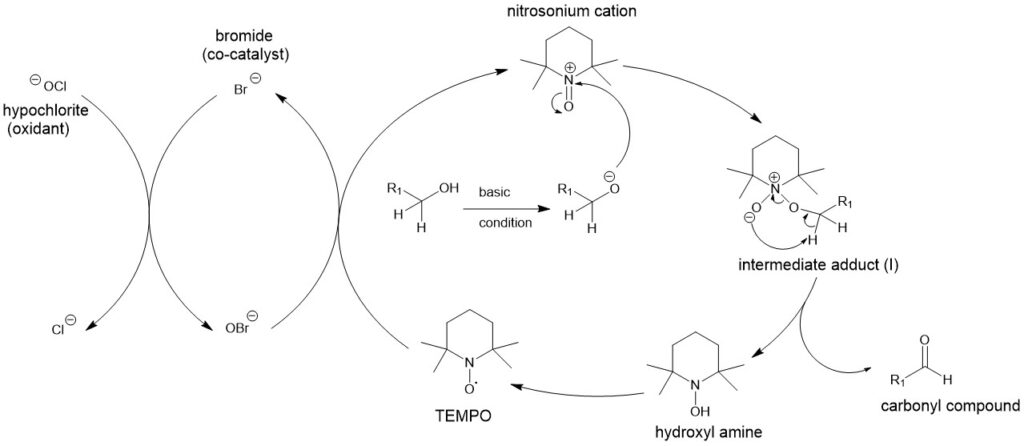
2,2,6,6-TETRAMETHYL-1- PIPERIDINYLOXY (TEMPO):
TEMPO and related stabilized nitroxyl radicals have found wide application as catalysts in green oxidation technology for the synthesis of aldehydes and ketones. The primary alcohols can be oxidized to aldehydes or carboxylic acids by using a catalytic amount of such stable nitrosyl radicals. 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) is one such nitrosyl radical for this reaction. Under the same conditions, secondary alcohols can be oxidized to ketones.
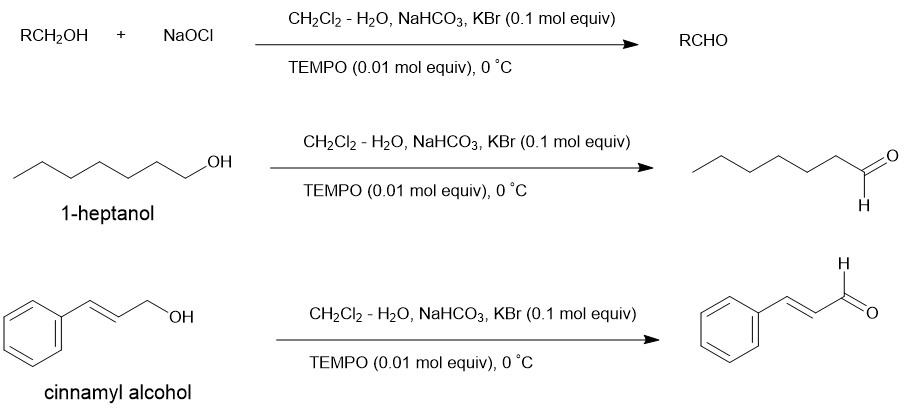
The oxidation of alcohols to carbonyl derivatives is, normally, carried out at 0 °C in CH2Cl2 – 0.35 M aqueous NaOCl (1.25 mol equivalent) in the presence of 0.01 mol equivalent of TEMPO. Besides, the addition of 0.10 mol equivalent of KBr and buffering with aqueous NaHCO3 is also required. Under these circumstances, oxidation to carboxylic acids does not occur.
Eg.
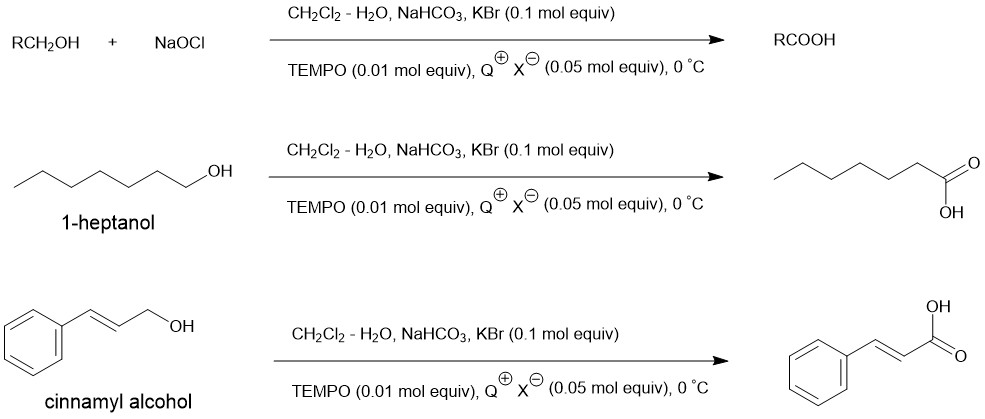
Under standard conditions, the addition of a catalytic amount of quarternary ammonium salts (eg. Methyltrioctylammonium chloride) leads to the oxidation of primary alcohols or aldehyde to the corresponding carboxylic acids.
MECHANISM:
Under homogeneous conditions, the oxidation of alcohols to carbonyl derivatives under the two-phase conditions involves the formation of an intermediate adduct (I). Subsequent proton abstraction gives the carbonyl compound and the hydroxylamine.
A large number of TEMPO variants exist based on the 2, 2, 6, 6,-tetramethylpiperidine motif. In recent years, several structurally diverse nitroxide radical oxidation catalysts have appeared. A drawback is the lack of commercial availability, although more are becoming commercial or readily prepared. A key feature is that these catalysts often show positive rate enhancements compared to TEMPO – often several orders of magnitude. The mechanism of action is identical, requiring a terminal oxidant like NaOCl to drive the catalytic cycle.