The generation of a carbocation followed by the [1,2]-shift of an adjacent carbon-carbon bond to generate a new carbocation is known as the Wagner–Meerwein rearrangement. It is named after the German chemist Hans Meerwein and Fritz Wagner, who independently discovered and studied the rearrangement in the early 20th century.
The Wagner-Meerwein rearrangement is closely related to carbocation chemistry. Carbocations are reactive intermediates with a positively charged carbon atom, and their rearrangement can lead to the formation of new carbon-carbon bonds or the rearrangement of substituents. The rearrangement was first observed and reported by Fritz Wagner in 1914 during his studies on the rearrangements of terpenes. A few years later, Hans Meerwein further investigated and expanded the scope of the reaction, leading to its recognition as the Wagner-Meerwein rearrangement.
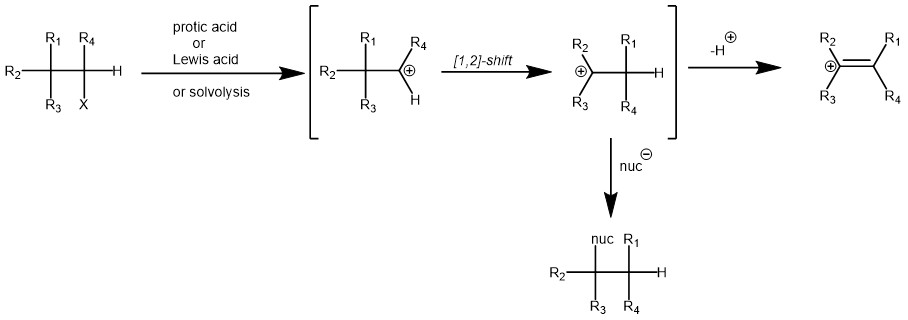
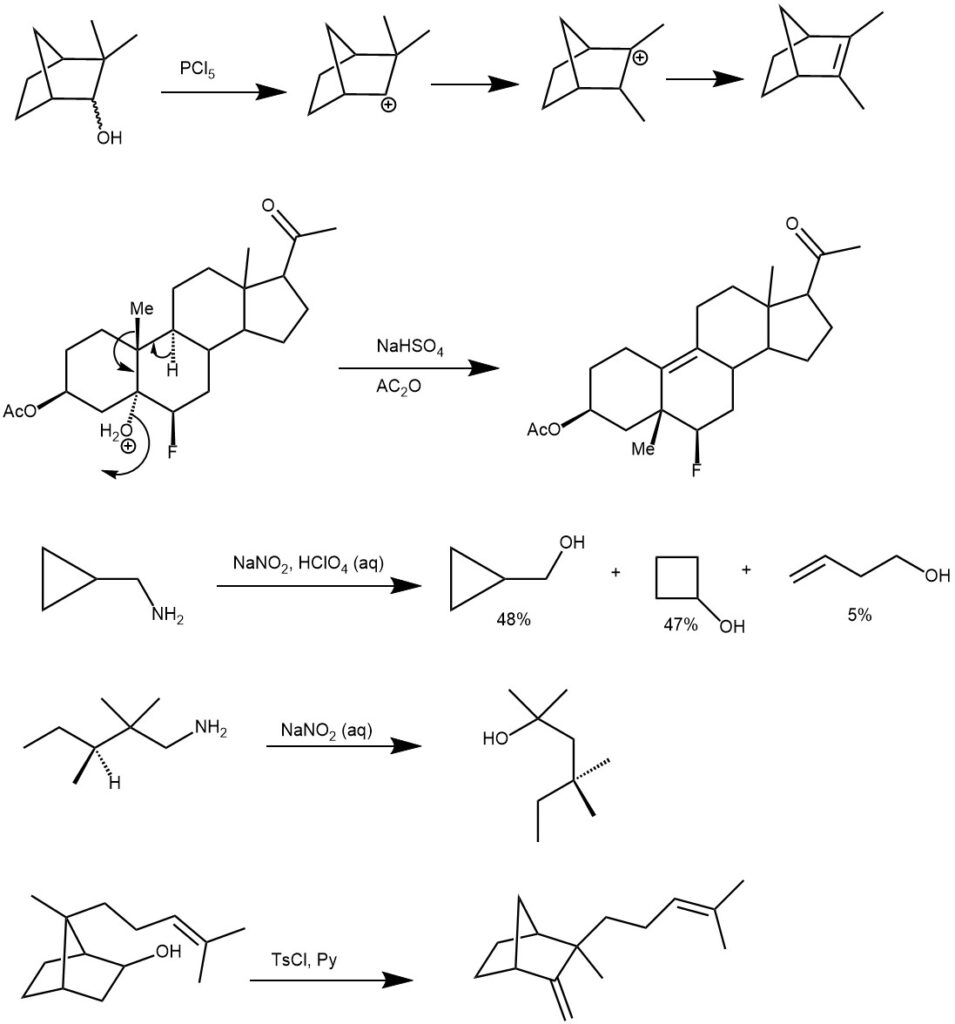
Importantly, the initial carbocation can be generated in a variety of ways like protonation of alkenes, alcohols, epoxides, or cyclopropanes, solvolysis of secondary or tertiary alkyl halides, deamination of amines with nitrous acid, etc. The initial carbocation can then undergo [1,2]-alkyl, aryl, or hydride shift to generate more stable carbocation. The final carbocation can then lose a proton to afford an alkene or react with a nucleophile.
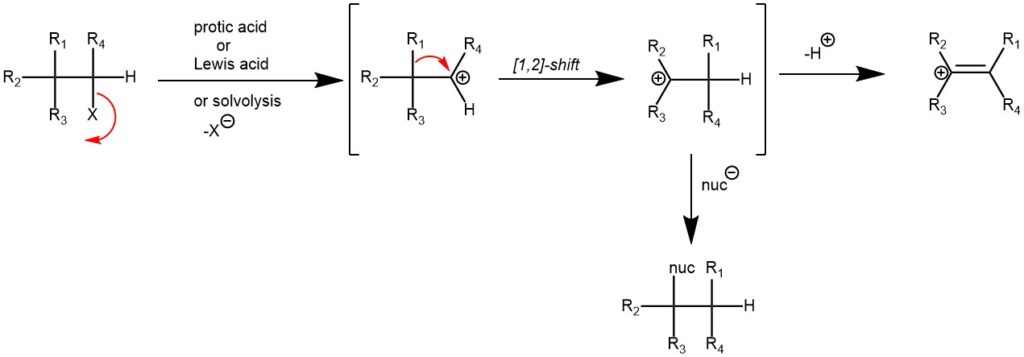
MECHANISM: The Wagner-Meerwein rearrangement proceeds through a concerted or stepwise mechanism, depending on the specific reaction conditions and substrates involved. In both cases, the reaction involves the migration of a carbon or hydride group from a carbocation intermediate to an adjacent carbon atom, resulting in a rearranged product. The rearrangement can occur in various organic compounds, including alcohols, ethers, alkenes, and alkyl halides.
CONCERTED MECHANISM:
In the concerted mechanism, the rearrangement occurs in a single step without the intermediacy of a discrete carbocation. The migrating group and the adjacent carbon atom undergo a simultaneous shift, leading to the rearranged product. The concerted mechanism is often observed in rearrangements of cyclic systems and involves a cyclic transition state.
STEPWISE MECHANISM:
In the stepwise mechanism, the carbocation intermediate is formed first, followed by the migration of the carbon or hydride group. The rearrangement can proceed through a 1,2-hydride shift, a 1,2-alkyl shift, or a combination of both. The stability and reactivity of the carbocation intermediate and the stability of the resulting rearranged product play crucial roles in the efficiency and outcome of the reaction.
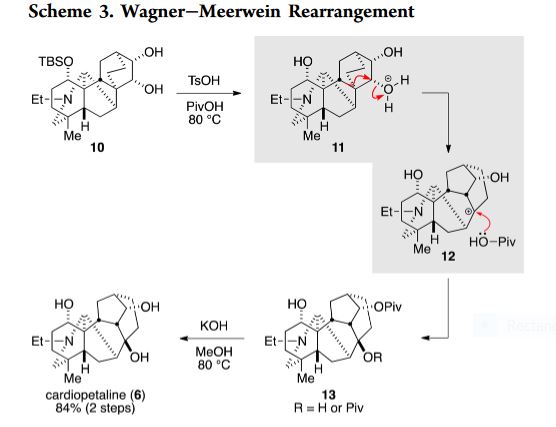
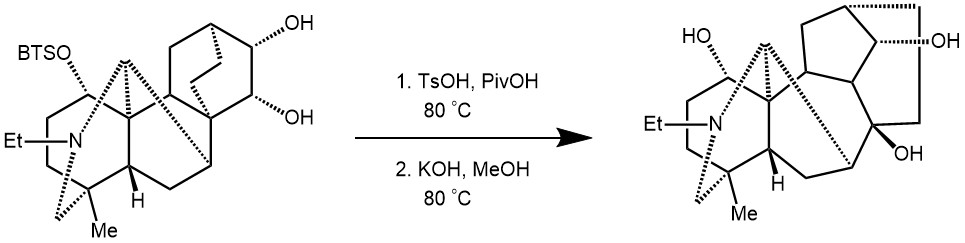
EXAMPLE 1: To a solution of (9.5 mg, MW = 461.8, 21 μmol) in pivalic acid (0.51 mL) was added p-toluenesulfonic acid (98 mg, MW = 190.2, 0.52 mmol). After heating at 80 °C for 150 min, aqueous sodium hydroxide (1 M) was added to the reaction mixture, and the mixture was extracted with dichloromethane. The aqueous phase was extracted twice with dichloromethane. The combined organic phases were dried over sodium sulfate, filtered, and concentrated. This material was used for the next step without further purification.
The residue was dissolved in potassium hydroxide solution (1.0 M in methanol, 0.51 mL) and the resulting solution was heated at 80 °C for 4 h in a sealed tube. After cooling to room temperature, water was added to the reaction mixture, and the mixture was extracted with dichloromethane. The aqueous phase was extracted twice with dichloromethane. The combined organic phases were dried over sodium sulfate, filtered, and concentrated. The residue was purified by preparative thin layer chromatography on amino silica gel eluting with ethyl acetate to give (cardiopetaline, 6.0 mg, MW = 347.5, 17 μmol, 84% in two steps) as a white solid. [REF: Org. Lett. 2017, 19, 5833−5835]
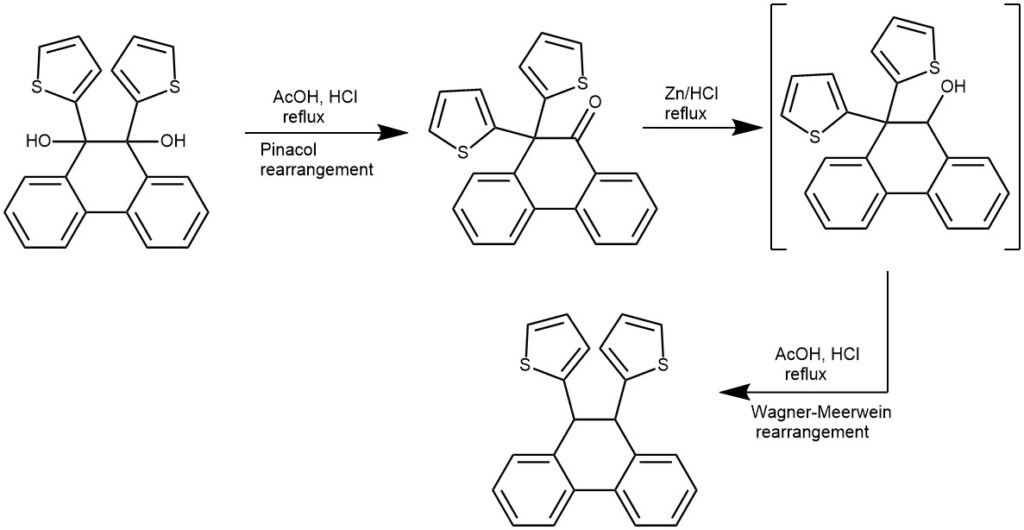
EXAMPLE 2: Into a refluxing solution of the diol (0.494 mmol, 1.0 eq) in AcOH (10 mL) was added Zn powder (162 mg, 2.47 mmol, 5.0 eq). Immediately conc. HCl (0.10 mL, 1.20 mmol, 2.4 eq) in AcOH (0.4 mL) was added dropwise. Half an hour later, another portion of zinc powder (162 mg, 2.47 mmol, 5.0 eq) and conc. HCl (0.10 mL, 1.20 mmol, 2.4 eq) in AcOH (0.4 mL) was added. The mixture was refluxed until no further reaction was indicated by TLC (1-3 h). The mixture was poured into 50 mL water and extracted with dichloromethane (50 mL x 3). Combined organic layers were washed with saturated aq. NaHCO3 and brine, and then dried with anhydrous MgSO4. Evaporation of solvent followed by column chromatographic purification on silica gel with petroleum ether and dichloromethane as eluent yielded the target product as a white solid. [REF: Org. Lett., Vol. 10, No. 5, 2008, 773-776]
REFERENCES:
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako
- https://www.sciencedirect.com/topics/chemistry/wagner-meerwein-rearrangement



