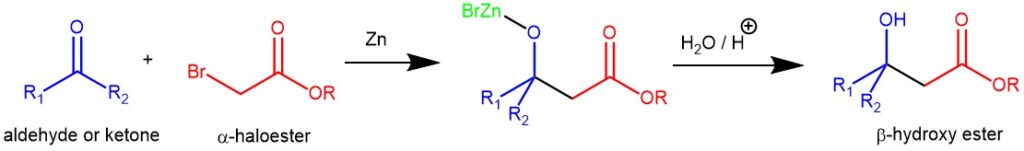
The classical Reformatsky reaction is defined as the zinc-induced reaction between an α-halo ester and an aldehyde and ketone. In this reaction, an α-halo ester (usually an α-bromo ester) is reduced with zinc to form a halozinc enolate in the presence of an aldehyde or ketone.
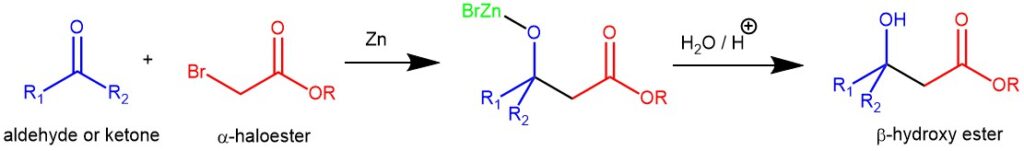
The reaction is a two-stage process: first the activated zinc metal inserts into the carbon-halogen bond, and this is followed by the reaction of the zinc enolate with the carbonyl compound in an aldol-type reaction.
Activated zinc metal can be generated in two different manners. The first method involves removing the deactivating zinc oxide layer from the metal surface using reagents like iodine, 1,2-dibromoethane, or copper(I) halides. The second method involves reducing zinc halides in solution using various reducing agents such as potassium, sodium, or lithium naphthalide. Other metals like lithium, magnesium, and iron can also be employed instead of zinc in this reaction.
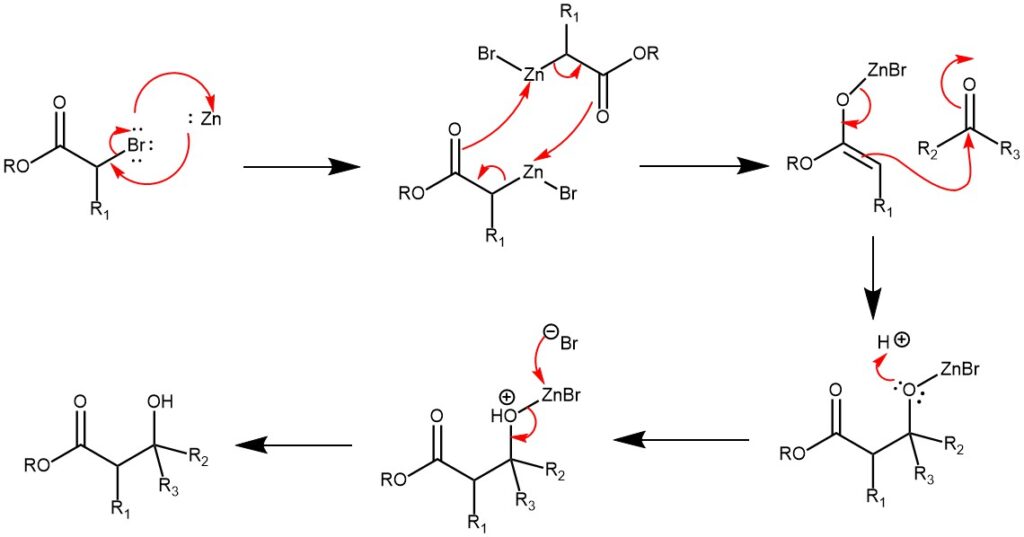
MECHANISM: The zinc metal is inserted into the carbon-halogen bond of the α-halo ester by a process called Oxidative addition. This compound dimerizes and rearranges to form two zinc enolates. The oxygen on an aldehyde or ketone coordinates with the zinc to form the six-membered chair-like transition state. A rearrangement occurs in which zinc switches to the aldehyde or ketone oxygen and a carbon-carbon bond is formed. Acid workup gives the β-hydroxy ester.
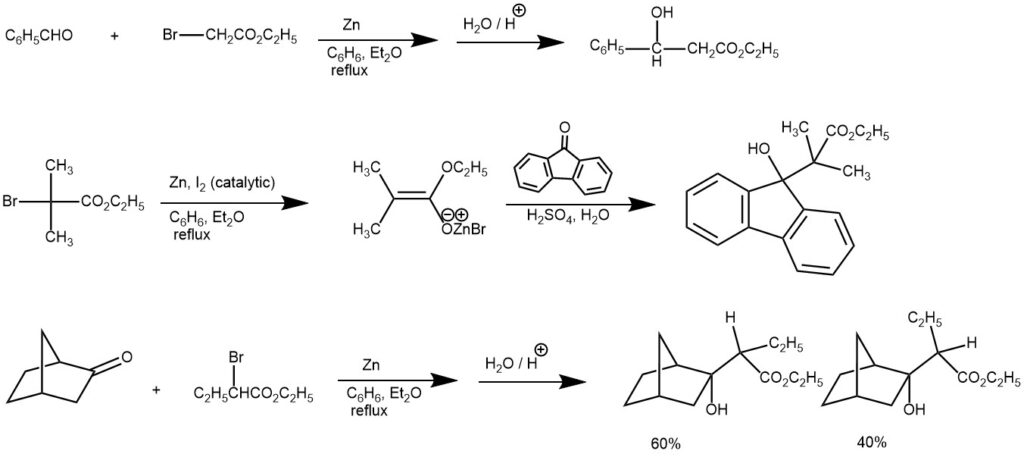
EXAMPLE 1: To a 500 mL round-bottom flask were added zinc powder (20 g) and aqueous 0.1 N aq. HCl (200 mL). The suspension was stirred vigorously for 10 min. The precipitate was collected by filtration and dried in vacuo at 100 °C for at least 4 h. Under N2 atmosphere, to a 200 mL round-bottom flask were added the above-activated zinc (11.5 g, 175.8 mmol, 2.0 equiv) and dry THF (44 mL). The suspension was warmed to 40−50 °C, and ethyl bromoacetate (14.7 g, 88.2 mmol, 1.0 equiv) in THF (110 mL) was added dropwise. After insoluble matter precipitated, the light-yellow supernatant solution was decanted and used for subsequent experiments.
To a 100 mL round-bottom flask were added ca. 0.54 mol/L of ethyl bromozincacetate/THF solution (23.3 mL, ca. 12.5 mmol, 5 equiv), TMEDA (0.75 mL, 5 mmol, 2.0 equiv), and carbonyl compound (2.5 mmol, 1.0 equiv). The solution was warmed to 50 °C and stirred for 1−5 h. After being cooled to room temperature, the mixture was diluted with EtOAc (50 mL). The solution was successively washed with 20% aq citric acid (25 mL), 10% aq NaCl (25 mL), 5% aq NaHCO 3 (25 mL), and water (25 mL). The organic layer was concentrated in vacuo to give the crude oil. The crude oil was purified by flash chromatography (EtOAc/hexane) to give the pure δ-hydroxy-β-ketoesters.[REF: | J. Org. Chem. 2013, 78, 5843−5850]
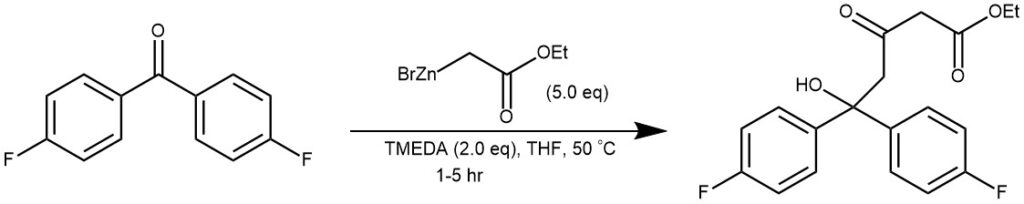
EXAMPLE 2: In a clean, dry, 500-ml. three-necked flask, fitted with a mechanical stirrer, a 250-ml. separatory funnel, and a reflux condenser, the upper end of which is protected by a calcium chloride drying tube, is placed 40 g. (0.62 gram atom) of powdered zinc.
A solution of 83.5 g. (0.50 mole) of ethyl bromoacetate and 65 g. (0.61 mole) of benzaldehyde in 80 ml. of dry benzene and 20 ml. of absolute ether is placed in the separatory funnel. About 10 ml. of this solution is added to the zinc, and the flask is warmed until the reaction starts. The mixture is then stirred, and the rest of the solution is added at such a rate that the reaction mixture refluxes, care being taken that the reaction does not become too vigorous. The addition should take about an hour. The reaction mixture is refluxed for 30 minutes on a water bath after the addition of the solution is complete.
The flask is then cooled in an ice bath and the reaction mixture is hydrolyzed by the addition of 200 ml. of cold 10% sulfuric acid with vigorous stirring during the addition. The acid layer is drawn off and the benzene solution is extracted twice with 50 ml. portions of 5% sulfuric acid. The benzene solution is washed once with 25 ml. of 10% sodium carbonate solution, then with 25 ml. of 5% sulfuric acid, and finally with two 25 ml. portions of water. The combined acid solutions are extracted with two 50-ml. portions of ether and the combined ether and benzene solutions are dried with 5 g. of magnesium sulfate or Drierite. The solution is filtered, the solvent is removed by distillation at atmospheric pressure from a steam bath, and the residue is fractionated under reduced pressure. The ester is collected at 151–154°/11–12 mm. (128–132°/5–7 mm.). The total yield is 59–62 g. (61–64%). [REF: Organic Syntheses, Coll. Vol. 3, p.408 (1955); Vol. 21, p.51 (1941)]
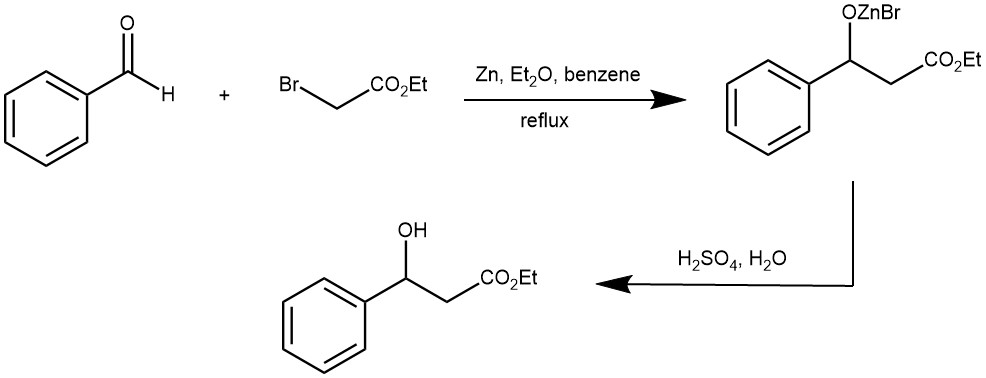
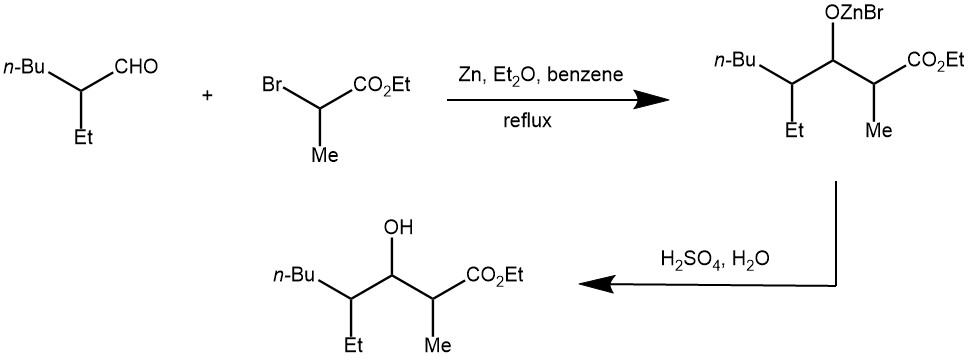
EXAMPLE 3: A 3L three-necked flask is equipped with a mechanical, a condenser arranged for distillation, and a 500-ml. pressure-equalizing dropping funnel. The flask is heated on a steam cone, and a slow stream of nitrogen is introduced from a cylinder through a line connected to the top of the dropping funnel. To the flask is added 98.1 g. (1.50 g. atoms) of freshly sandpapered zinc foil which has been cut into narrow strips and rolled loosely, and 750 ml. of thiophene-free benzene previously dried over sodium.The benzene is heated to reflux and a solution of 64.1 g. (0.50 mole) of 2-ethylhexanal and 271.5 g. (1.50 moles) of ethyl α-bromopropionate in 500 ml. of dried benzene is placed in the dropping funnel. The first 50 ml. of the solution is added to the flask at once. Usually reaction begins immediately, as evidenced by darkening of the zinc surface and clouding of the solution, but in some cases as much as 15 minutes elapses before the start of reaction. When the reaction has started, the remainder of the aldehyde-bromo ester solution is added during 1 hour, with stirring, as the solution is maintained under reflux. After addition is complete the mixture is heated for an additional 2 hours under reflux, and then cooled to room temperature.
The nitrogen line is removed, and to the solution is added 750 ml. of 12N sulfuric acid; the resulting mixture is stirred vigorously for 1 hour, and then decanted into a 3L. separatory funnel. After the two phases have separated, the lower aqueous layer is drawn off into a 3L. separatory funnel containing 1L of water which has been used to wash the reaction flask, and the diluted mixture is extracted twice with 350 ml. portions of benzene which have also been employed to rinse the reaction flask. The original organic layer and the combined benzene extracts are kept separate and are washed successively with 500 ml. portions of water, saturated sodium bicarbonate solution, and again with water. The two organic portions are now combined, allowed to stand over anhydrous sodium sulfate until clear, then transferred to a 2L distilling flask, from which solvent is distilled at atmospheric pressure, last traces under aspirator pressure. The residue remaining in the flask weighs 150–165 g. (Note 4) and is dehydrated without further purification. [REF: Organic Syntheses, Coll. Vol. 4, p.444 (1963); Vol. 37, p.37 (1957)]
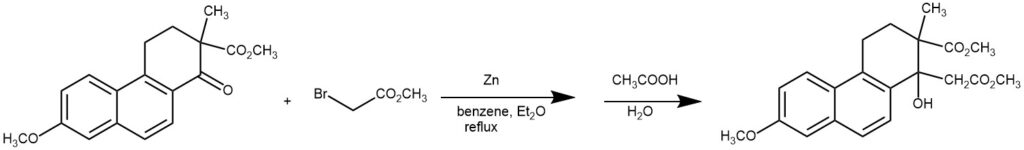
EXAMPLE 4: To 2.5 g. of granulated zinc (20-mesh, previously washed with dilute hydrochloric acid, water, acetone and dried) and 0.07 g. of iodine in 25 cc. of dry benzene (thiophene-free) and 25 cc. of anhydrous ether was added 1.5 g. of the keto ester and 0.75 cc. of methyl bromoacetate. As the mixture was refluxed on a water bath, the iodine color faded and the solution became cloudy. After five to ten minutes a colorless addition product was deposited. Five additions of 2.5 g. of zinc and a trace of iodine were made at forty-five-minute intervals and an additional 0.75 cc. of methyl bromoacetate was introduced after one and one-half hours. The mixture was refluxed for a total of four hours. Frequently the mixture was shaken vigorously in order to free the zinc from the adhering mass of crystals.
The addition product was dissolved by adding a little acetic acid and methanol and the solution was decanted from the zinc into water and the mixture acidified with acetic acid. The ether-benzene layer was separated, the aqueous solution was extracted with benzene and the combined extracts were washed with water and then dilute ammonium hydroxide until no more color was removed. The residue obtained by evaporation of the ether-benzene solution crystallized readily from methanol; yield 1.5-1.6 g. of colorless crystals (m. p. 123-126°). Additional product from the filtrate brought the total yield up to 85-90%. Should the yield fall below this, retreatment of the material in the filtrates with zinc and methyl bromoacetate will give additional product. The hydroxy ester crystallizes from methanol containing a few drops of acetone in colorless leaflets; m. p. 125-125.5°. With concentrated sulfuric acid the compound gives a blue color which soon changes to a red-brown color and then fades to yellow.[REF: W. E. Bachmann, W. Cole, and A. L. Wilds, J. Am. Chem. Soc., 62, 824 (1940)]
REFERENCES:
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako
- Modern synthetic reactions by Herbert O. House