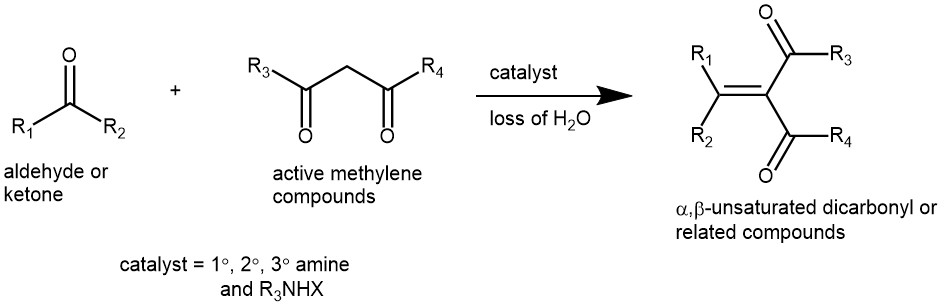
Knoevenagel condensation is a nucleophilic addition of an active methylene compound to a carbonyl group followed by a dehydration reaction in which a molecule of water is eliminated (hence condensation). Specifically, the reaction of aldehydes and ketones with active methylene compounds in the presence of a weak base to afford α,β-unsaturated dicarbonyl, or related compounds is known as the Knoevenagel condensation. The product is often an α,β-unsaturated ketone (a conjugated enone). It is an important condensation reaction in synthetic organic chemistry for the preparation of vital α,β-unsaturated compounds. It has been used for the synthesis of important chemical intermediates, pharmaceuticals, polymers, cosmetics, and perfumes.
SOME EXAMPLES
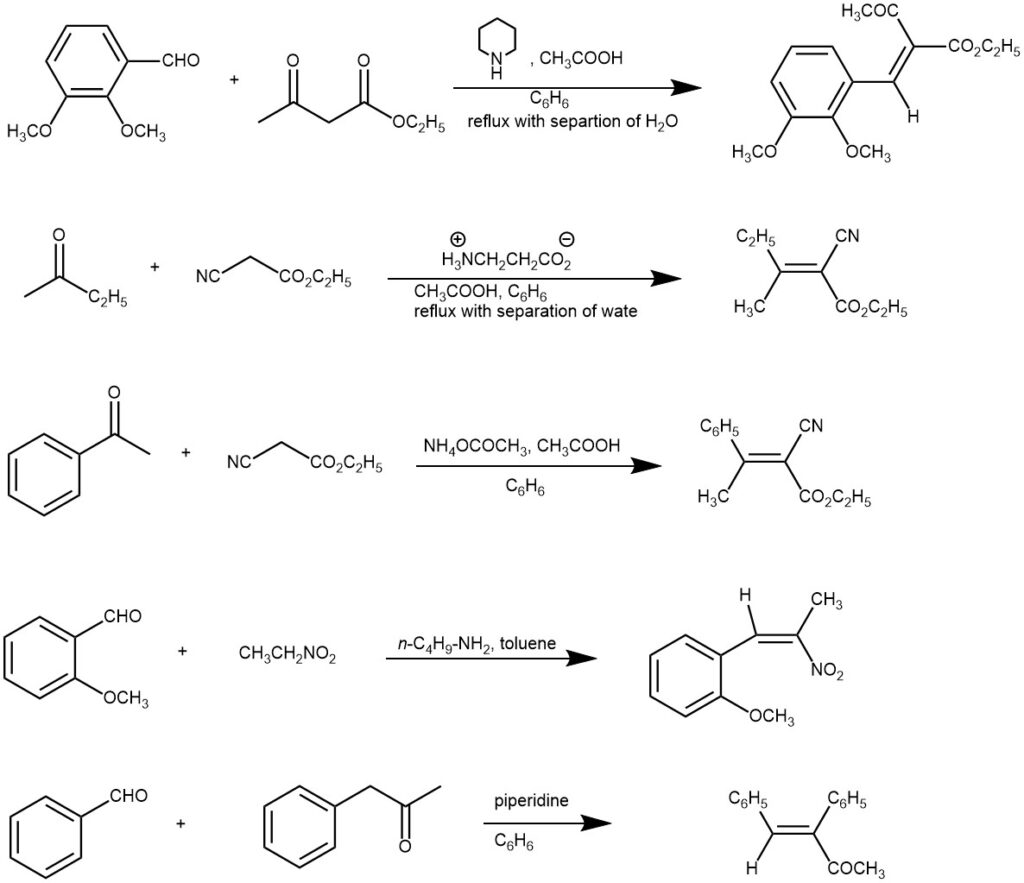
The active methylene compounds need to have two electron-withdrawing groups and typical examples are malonic esters, acetoacetic esters, malonodinitrile, acetylacetone, benzyl ketones, aliphatic nitro compounds, etc. The nature of the catalyst is important. Ammonia, or a primary or secondary amine, and at least a catalytic amount of carboxylic acid is mostly used. Various ammonium salts (e.g. ammonium acetate, piperidinium acetate) are frequently used to supply the necessary catalysts.
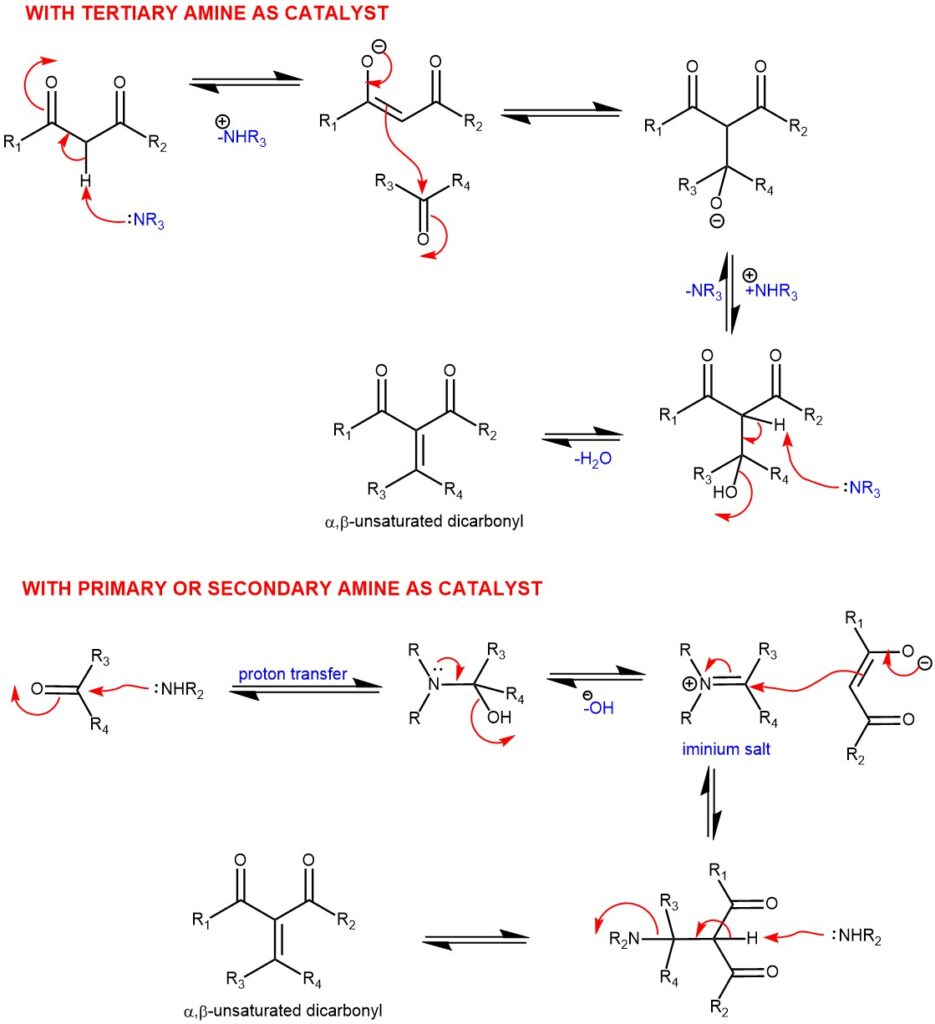
MECHANISM: The Knoevenagel condensation is a base-catalyzed Aldol–type reaction. When tertiary amines are used as catalysts, the formation of a β-hydroxydicarbonyl intermediate is expected, which undergoes dehydration to give the product. While using secondary or primary amines as catalyst leads to an iminium salt which reacts with the enolate and 1,2-elimination gives rise to the desired α,β-unsaturated dicarbonyl or related compounds.
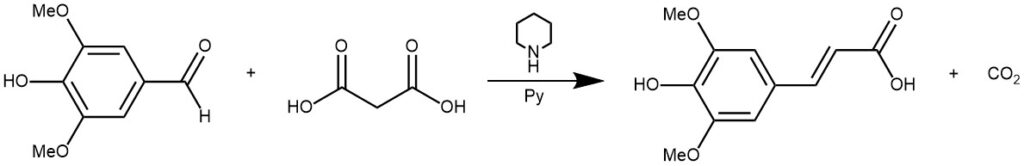
EXAMPLE 1: Malonic acid (1.0 g,10 mmol) was dissolved in pyridine (3.0 mL, 37 mmol). Syringaldehyde (1, 0.90 g, 5 mmol) and piperidine (0.20 mL, 2 mmol) were subsequently added. The reaction was heated to the desired temperature 70 °C. After 3 hours, the reaction was stopped and pyridine was removed in vacuo. The residue was dissolved in a small amount of a saturated aqueous NaHCO3 solution and acidified to a pH of 2 with 6 M HCl. The resulting precipitate was separated by filtration and washed with water. After recrystallization with a solution of water- ethanol (v/v) (3:1), the crystals were separated by filtration and dried in a nitrogen atmosphere to get a yield of 78%.[REF: Open Journal of Physical Chemistry, 2016, 6, 101-108]
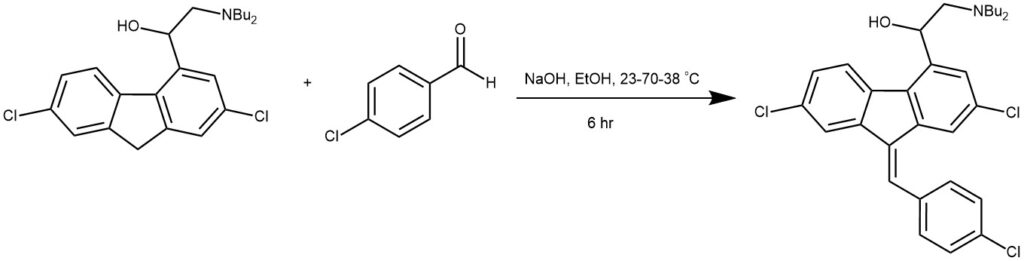
EXAMPLE 2: A stirred mixture of 8 (80 g, 0.197 mol), p-chlorobenzaldehyde (29 g, 0.207 mol), sodium hydroxide (15.8 g, 0.394 mol), and ethanol (1160 mL) was heated up within 1 h from 23 to 70 °C and immediately afterward cooled within 1 h from 70 to 38 °C. The mixture was agitated at 38 °C for at least 4 h to complete crystallization. After filtration, washing, and drying, 91.6 g (88%) of crude 2 was isolated. A mixture of crude 2 (15 g, 0.284 mol) and heptane fraction (75 mL) was heated to reflux (94 °C). A solution was formed which was clear filtered above 90 °C. The solution was cooled to 70 °C, seeded with pure 2, cooled within 4 h to 3 °C, and stirred at that temperature for at least 1 h. The suspension was filtered, washed, and dried to yield 13.9 g (93%) of pure 2.[REF: Organic Process Research & Development 2007, 11, 341−345]
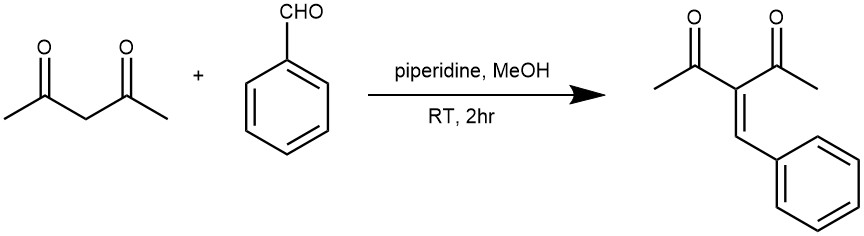
EXAMPLE 3: To a solution of acetylacetone (0.51 mL, 0.50 g, 5.0 mmol) and benzaldehyde (0.51 mL, 0.53 g, 5.0 mmol) in methanol (10.0 mL) was added piperidine (0.05 mL, 0.043 g, 0.5 mmol) at room temperature. After 2 h of reaction, the reaction mixture was diluted with ethyl acetate (EtOAc) (10.0 mL) and saturated aqueous sodium chloride (5.0 mL). The layers were separated and the aqueous layer was extracted with EtOAc (2x 10.0 mL). The organic extracts were combined, dried over sodium sulfate, and evaporated to dryness under a vacuum. The residue obtained was purified by chromatography on silica gel flash (EtOAc/Hexane 3:7). The product was isolated as being an orange viscous liquid in 80% yield.[REF: J. Phys. Chem. B 2017, 121, 5300−5307]
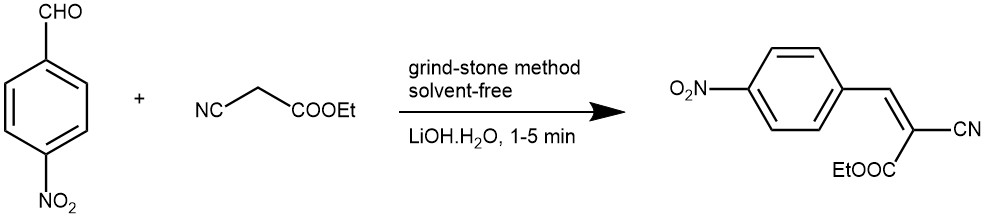
EXAMPLE 4: A mixture of an aldehyde (1 mmol), active methylene compound (1 mmol), and lithium hydroxide (0.1 mmol) were ground together using a pestle and a mortar at 26 °C. The reaction mixture got solidified within 1–5 min. After completion of the reaction (TLC), water was added, stirred for a minute then filtered and dried. Recrystallization was not necessary.[REF: Journal of Saudi Chemical Society (2011), 15, 283-286]
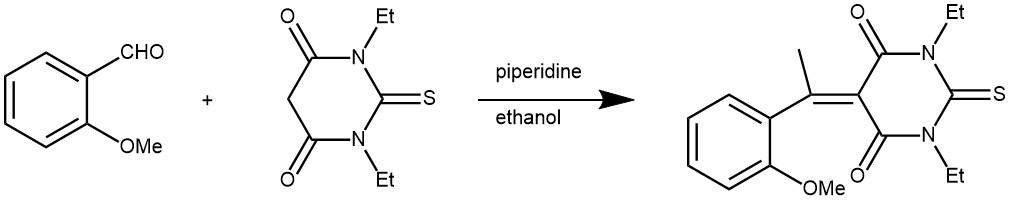
EXAMPLE 5: N,N-diethylthiobarbituric acid (1.50 g, 7.5 mmol) and 2-methoxybenzaldehyde (1.02 g, 0.02 mmol) in ethanol (30 mL) were heated under reflux for 5 minutes. Piperidine (0.5 mL) was added in one portion and the reflux was continued for two hours. The reaction mixture was cooled to room temperature and the solid formed was filtered, washed with cold ethanol and dried. It was then recrystallized from ethanol to yield deep yellow crystals (2.03 g, 85%)[REF: Molbank 2004, M359]
REFERENCES:
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako
- Modern synthetic reactions by Herbert O. House