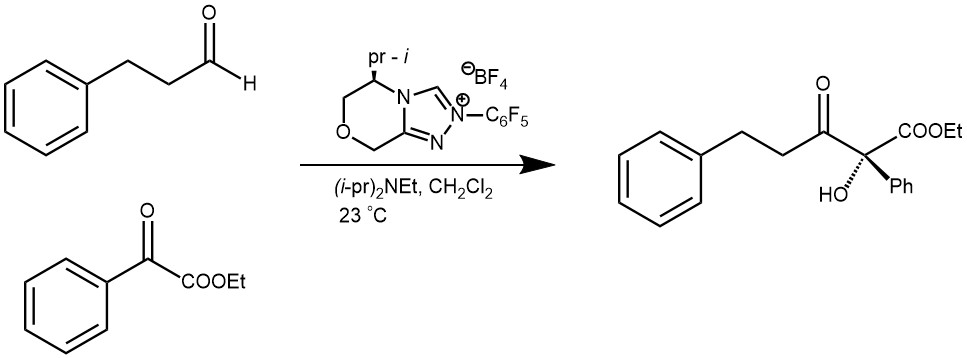
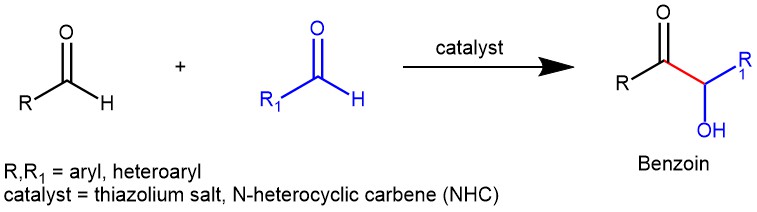
The benzoin reaction, also known as benzoin condensation, is a chemical process named after the product it produces. It involves the combination of two aromatic aldehyde molecules with the help of a catalyst. One aldehyde acts as an acyl anion, while the other aldehyde acts as a carbon electrophile. This results in the formation of α-hydroxy ketones, specifically benzoins. There are two variations of this reaction: the homo-benzoin condensation, where two molecules of the same aldehyde are used, and the crossed-benzoin condensation, where two different aldehydes are used. Two main classes of catalysts are commonly used to facilitate benzoin condensation: alkali metal cyanides (such as NaCN and KCN) and N-heterocyclic carbenes (NHCs). The previous blog discussed the benzoin condensation catalyzed by cyanide ions. In contrast, this article will focus on the benzoin reaction catalyzed by N-heterocyclic carbenes (NHCs).
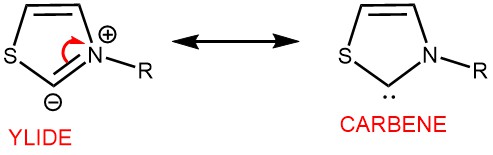
In general, thiazolium salt-derived NHCs have found widespread application as catalysts for benzoin condensation. However, triazolium and imidazolium-derived NHCs have also emerged as favorable ones.
MECHANISM:
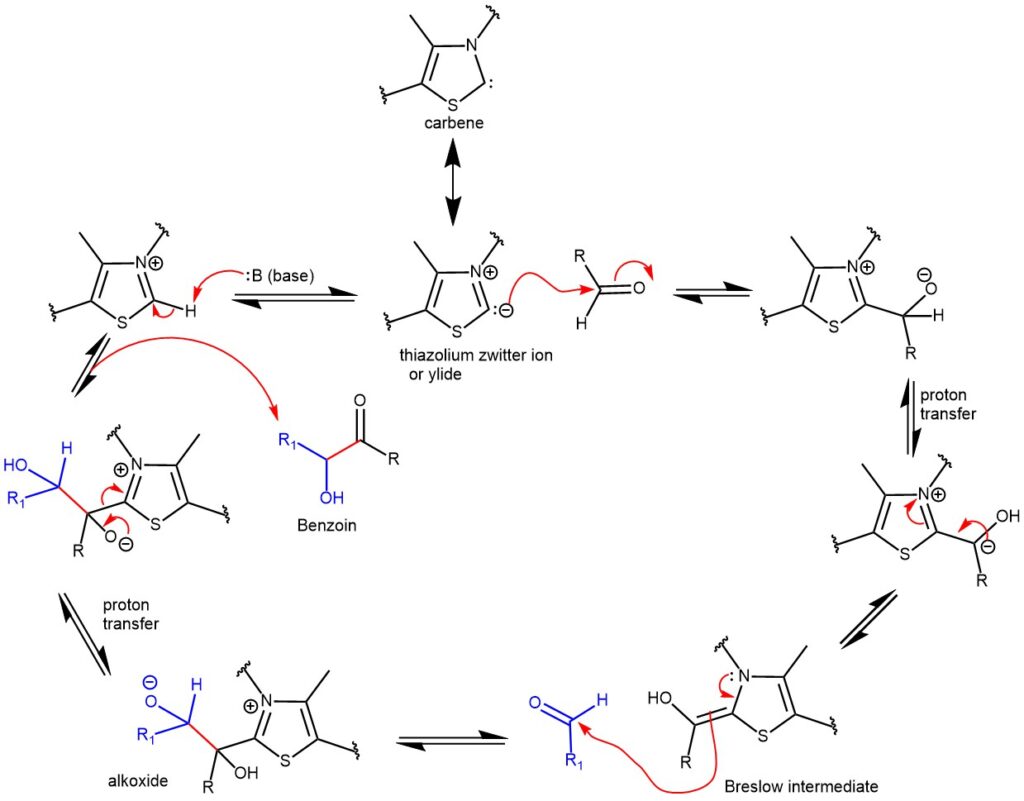
The benzoin reaction involves a polarity reversal process called umpolung, which is facilitated by the catalyst. This mechanism was initially proposed by Breslow in 1958 for the thiazolium salt-catalyzed benzoin reaction. The active species responsible for catalysis is a thiazolium zwitterion, which is a resonance structure of an N-heterocyclic carbene (NHC). The reaction proceeds through an intermediate known as the “Breslow Intermediate,” which is an enaminol compound.
To initiate the reaction, the thiazolium salt is deprotonated by a suitable base, forming a nucleophilic thiazolium zwitterion or ylide. This ylide can be represented as a resonance structure of a carbene. The ylide then undergoes nucleophilic addition to the aromatic aldehyde, resulting in the formation of a tetrahedral intermediate. Proton transfer subsequently occurs, leading to the formation of an enaminol derivative. The enaminol derivative alters the polarity of the aldehyde carbon, making it nucleophilic. This nucleophilic carbon then reacts with another molecule of aldehyde, forming an alkoxide intermediate. Proton transfer takes place once again, followed by the release of the catalyst, ultimately yielding the benzoin product.
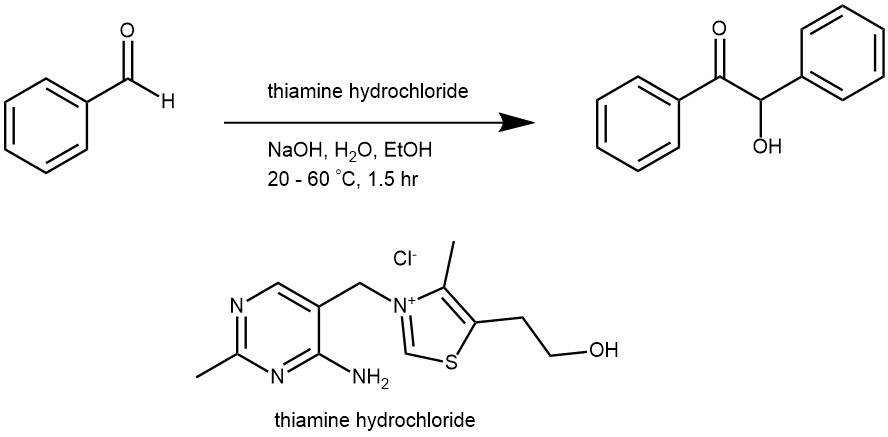
EXAMPLE 1: Add 1.30 g of thiamine hydrochloride to a clean 50-mL R.B. flask, and add a stir bar. Dissolve the solid in 4.0 mL of water by stirring. Add 15 mL of 95% ethanol and cool the solution for a few minutes in an ice bath. Slowly, add 2.5 mL of cold 3 M NaOH dropwise, making certain that the temperature of the solution never rises above 20 °C. To the yellow solution, add 7.5 mL of pure benzaldehyde. Equip the flask with a reflux condenser and heat the mixture at 60 °C for about 1.5 hours. Cool the reaction mixture in an ice bath. If crystallization does not occur immediately, withdraw a drop of the solution on a stirring rod and let it dry to produce a solid; then, rub it against the inside surface of the flask to induce crystallization. Collect the product by vacuum filtration and wash it free of any yellow mother liquor with a cold 1:1 mixture of 95% ethanol and water. It is in your best interest to recrystallize the moist product from 95% ethanol (~8 mL of ethanol per gram of product). The product should be colorless and of sufficient purity (mp 134-135 oC) to use in subsequent reactions. The usual yield is about 5 – 6 g.
EXAMPLE 2: The triazolium salt (6.7 mg, 0.017 mmol), methyl-4-formylbenzoate (58 mg, 0.35 mmol), and internal standard bibenzyl (32 mg, 0.18 mmol) were added to a test tube with a Schlenk take-off which was then fitted with a septum and placed under inert atmosphere. Hydrocinnamaldehyde (69 μL, 0.52 mmol), and CH2Cl2 (0.35 mL, 1 M) were added. Lastly (i-Pr)2NEt base was added (61 μL, 0.35 mmol) and the septum was then quickly exchanged for a cold finger. Once the inert atmosphere was reestablished the flask was sealed and heated to 70 °C for 3 hours, then quenched with acetic acid (100 μL). The crude reaction mixture was immediately purified by column chromatography (25% EtOAc/Hex) to yield 64 mg (61% yield) of the cross-benzoin product as pale yellow oil. Rf = 0.3 (25%EtOAc/Hex)[REF: J. Am. Chem. Soc. 2014, 136, 7539−7542]
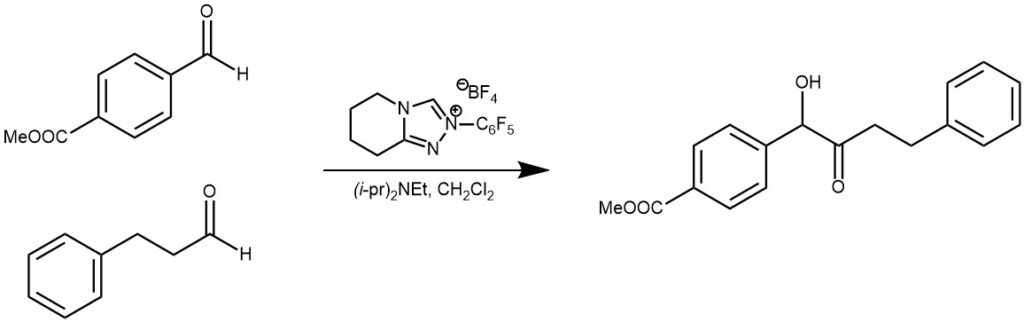
EXAMPLE 3: Aliphatic aldehyde (1.5 equiv) was added to a suspension of the appropriate α-ketoester (1 equiv), (S)-5-isopropyl-2-(perfluorophenyl)-6,8-dihydro-5H‐[1,2,4]triazoleo[3,4-c]oxazin-2-ium tetrafluoroborate (0.1 equiv), and 4Å powered molecular sieves (1:1 w/w, α-ketoester/4Å MS) in anhydrous CH2Cl2 (0.2 M) at rt, under an inert atmosphere. After stirring at rt for 10 min, iPr2NEt (1 equiv) was added and the reaction was monitored by TLC. The reaction was quenched with AcOH (10 μL), and the reaction mixture was filtered through a short pipette column of silica (~1.5 inches), washed with EtOAc, then concentrated under reduced pressure. The crude product was purified by column chromatography to yield the cross-benzoin product. [REF: Org. Lett., Vol. 15, No. 9, 2013]