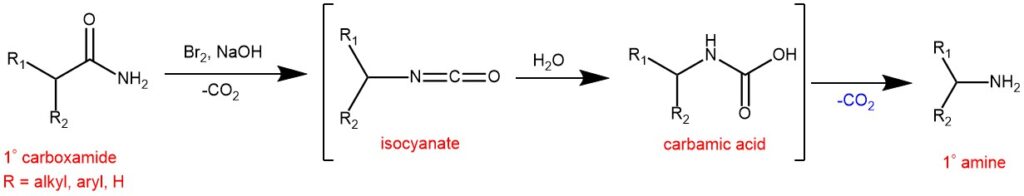
The Hofmann reaction (also called Hofmann degradation or Hofmann rearrangement) was previously reported in 1881 by the German chemist August Wilhelm Von Hofmann (1818–1892) and is the conversion of carboxamides (amide) into primary amines with one fewer carbon atom. The Hofmann rearrangement involves the conversion of an amide to an amine containing one fewer carbon atoms by treatment with bromine (or chlorine) and alkali (NaOH or KOH). According to the standard procedure, the amide is dissolved in a cold solution of an alkali hypobromite, or hypochlorite and the resulting solution is heated to ~70-80 0C to bring about the rearrangement. The hypohalite reagents are freshly prepared by the addition of chlorine or bromine to an aqueous solution of KOH or NaOH.
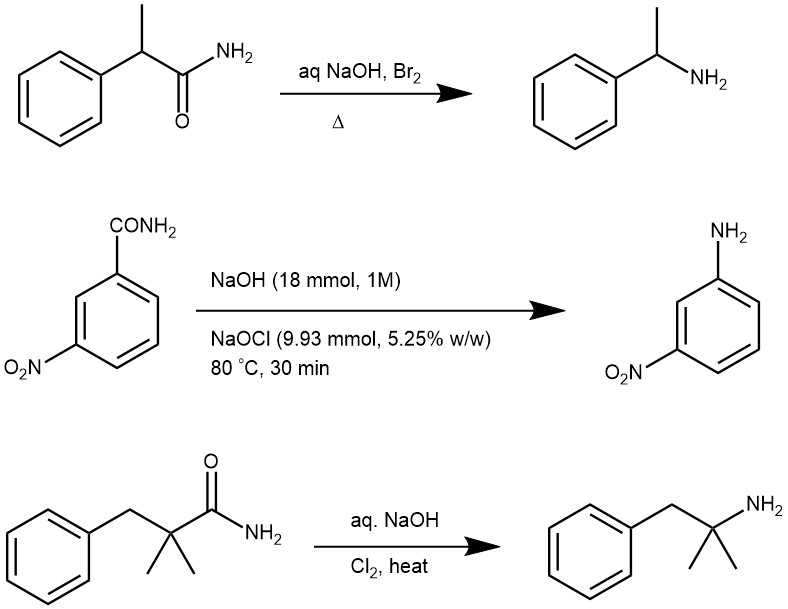
EXAMPLES:
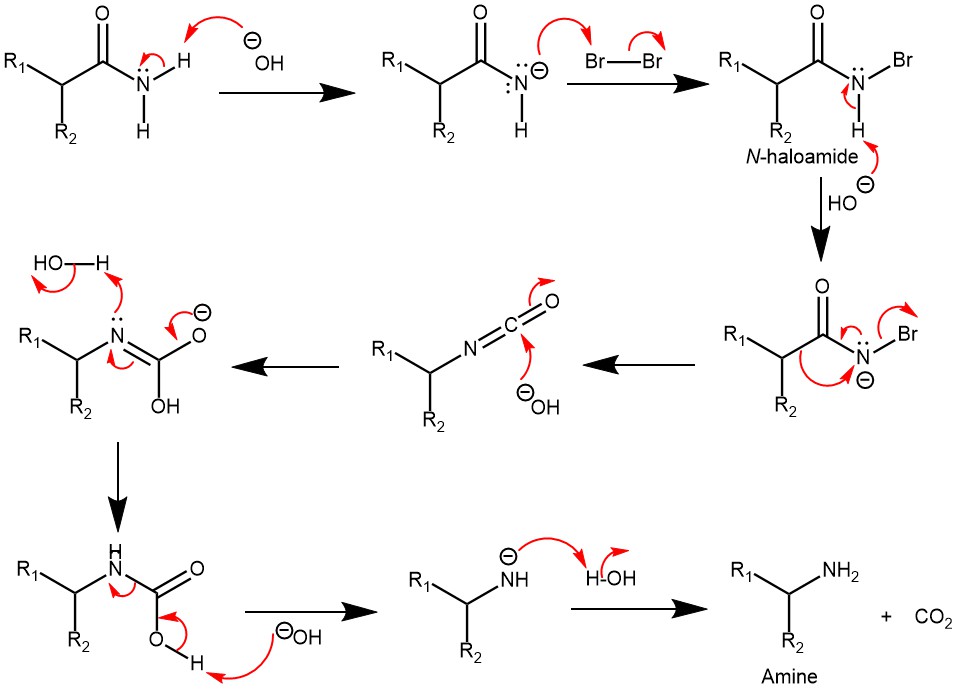
MECHANISM: Traditionally, the reaction is carried out in aqueous solutions using hypohalites or halogens/alkaline hydroxides and proceeds to rearrange the N-haloamide generated in situ to an isocyanate, followed by hydrolysis to a carbamate salt that further loses CO2 to give the primary amine.
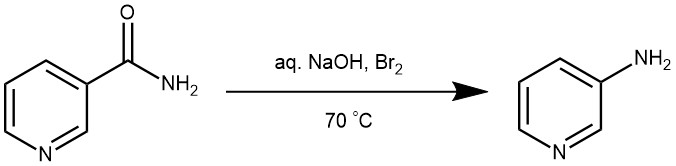
EXAMPLES 1: In a 2L beaker equipped with a mechanical stirrer and immersed in an ice-salt bath is placed a solution of 75 g. (1.87 moles) of sodium hydroxide in 800 ml. of water. To the solution is added, with stirring, 95.8 g. (30.7 ml., 0.6 mole) of bromine. When the temperature of the solution reaches 0°, 60 g. (0.49 mole) of nicotinamide is added all at once with vigorous stirring. After being stirred for 15 minutes, the solution is clear. The ice-salt bath is replaced by a bath containing water at 75°, and the solution is stirred and heated at 70–75 °C for 45 minutes. The solution is cooled to room temperature, saturated with sodium chloride (about 170 g. is required), and extracted with ether in a continuous extractor. The extraction time is 15–20 hours. The ether extract is adjusted to a volume of 1L., dried over 4–5 g. of sodium hydroxide pellets, and filtered, and the ether is removed by distillation from a steam bath. The residue crystallizes on cooling. The yield of dark red crystals melting at 61–63° is 39–41 g. (85–89%). The crude product is dissolved in a mixture of 320 ml. of benzene and 80 ml. of ligroin (b.p. 60–90°) and heated on a steam bath with 5 g. of Norit and 2 g. of sodium hydrosulfite for 20 minutes. The hot solution is filtered by gravity, allowed to cool slowly to room temperature, and then chilled overnight in a refrigerator. The product is isolated by gravity filtration (Note 3), washed on the filter with 25 ml. of ligroin, and dried in a vacuum desiccator. The yield of white crystals melting at 63–64° amounts to 28–30 g. (61–65%). By concentrating the combined filtrate and washings to a volume of 150 ml., an additional 2–3g. of pale yellow crystals melting at 62–64° can be obtained. The total yield of 3-aminopyridine is 30–33 g. (65–71%).[REF: Organic Syntheses, Coll. Vol. 4, p.45 (1963); Vol. 30, p.3 (1950).]
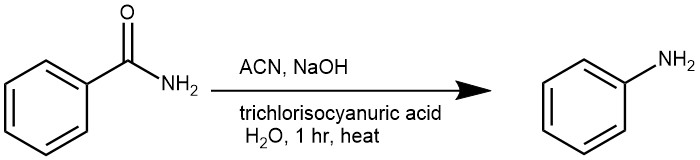
EXAMPLES 2: A solution of the carboxamide (20 mmol) and NaOH (4.4 g, 110 mmol) in H2O (15 mL) and MeCN (25 mL) was poured into a cold suspension of the trihaloisocyanuric acid (6.7 mmol) in water (10 mL), stirred for 1 h at 0 oC and then heated under reflux for 30 min. After cooling, the solid (cyanuric acid) was filtered off, and the filtrate was extracted with dichloromethane (3 x 20 mL). The combined layers were dried (Na2SO4) and the solvent evaporated to give the amine. [REF: Tetrahedron Letters 83 (2021) 153422]
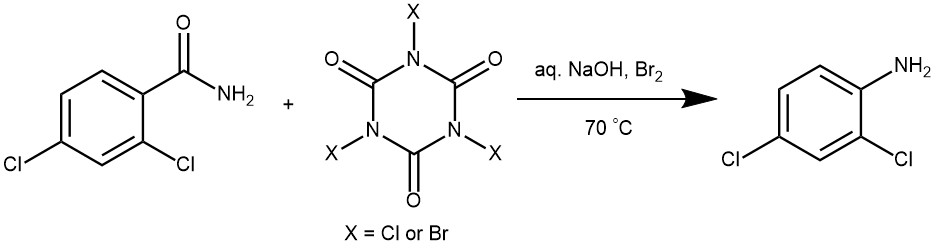
EXAMPLES 3: Place the reactant (330 mg, 0.5430 mM) in a 100 ml round bottom flask along with aqueous KOH solution (17ml, 5M). Add Br2, H2O solution (43.17 mL, 0.8415 mM) slowly to this reaction mixture. Stir the reaction mixture for 12h at 90 °C. Stop the reaction and cool it to room temperature. Add 20 mL ethyl acetate. Wash the organic solution with water (2 x 20 mL) followed by brine solution (20 mL). Dry the organics over anhydrous Na2SO4 Evaporate the organic solution.[ Langmuir (2014), 30(8), 1969-1976]
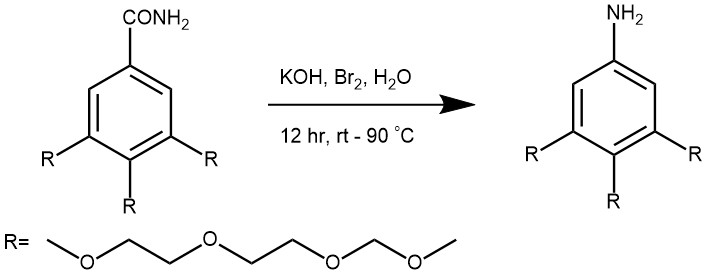
EXAMPLES 4: Suspend the benzamide (1.0 g, 8.4 mmol) in 1,4-dioxane (20.0 mL) as solvent. Mix the solution of NaOCl (10% active in chlorine, 12.4 mL, 20.2 mmol) with NaOH (50% aqueous solution, 1.7 mL, 20.2 mmol). Pump the amide slurry and the NaOCl solution separately, each at a rate of 1.3 mL min into the reactor. Run the reaction at 80 °C with a retention time of 15 minutes. [Organic Process Research & Development (2011), 15(5), 997-1009]
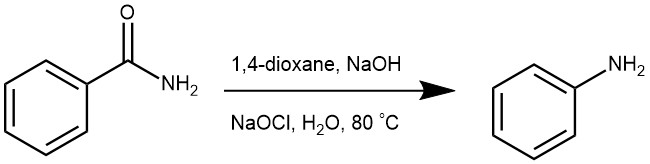
EXAMPLES 5: Pour a solution of the carboxamide (20 mmol) and NaOH (4.4 g, 110 mmol) in H2O (15 mL) and MeCN (25 mL) into a cold suspension of the trihaloisocyanuric acid (6.7 mmol) in water (10 mL). Stir the mixture for 1 h at 0°C and heat under reflux for 30 min. Cool the solution and filter the solid (cyanuric acid). Extract the filtrate with dichloromethane (3 x 20 mL) and dry the combined layers (Na2SO4). Evaporate the solvent to obtain the product [REF: Tetrahedron Letters (2021), 83, 153422]
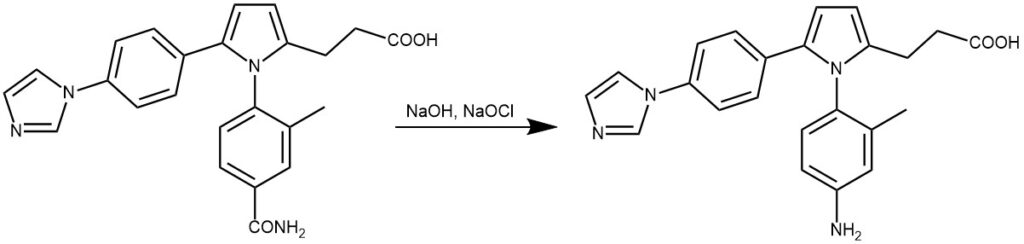
EXAMPLES 6: 3-(5-(4-(IH- imidazol-l-yl)phenyl)-l-(4-carbamoyl-2-methylphenyl)-lH-pyrrol-2-yl)propanoic acid (3.88 g, 9.37 mmol) was added to aq. NaOH (4.12 g, 103.09 mmol, dissolving in 50 mL). Then 11% aq. NaClO (28.83 g, 42.17 mmol) was added dropwise. The resulting mixture was kept at 0-1O 0C for 1h, at 35°C for 1h and at 75°C for 30min. After cooling to room temperature, the reaction was acidified with 10% hydrochloric acid to pH=7.0 and filtered to remove the solid impurity. The filtrate was further acidified with 10% hydrochloric acid to pH=5.0 and a new precipitate appeared. The precipitate was filtrated and dried to afford the product as a gray powder (3.20 g, 88%).[REF: World Intellectual Property Organization WO 2010/019903]
Reference:
- Modern Synthetic Reactions by Herbert O. House
- Strategic applications of named reactions in organic synthesis by Laszlo Kurti and Barbara Czako


