The Baeyer Villiger oxidation is an organic reaction that converts a ketone into an ester or a cyclic ketone into a lactone by breaking a carbon-carbon bond and inserting an oxygen atom. The reaction requires a peroxyacid or a peroxide as the oxidizing agent and can be catalyzed by a Lewis acid. The reaction is named after Adolf von Baeyer and Victor Villiger who first reported it in 1899. A wide range of oxidizing agents can be used to perform the Baeyer- Villiger oxidations and their activity is ranked as follows: peroxytrifluoroacetic acid (CF3COOOH) > monopermaleic acid > monoperphthalic acid > 3, 5-dinitroperbenzoic acid > p-nitroperbenzoic acid > mCPBA ~ performic acid > perbenzoic acid > peracetic acid >> hydrogen peroxide > t-BuOOOH (REF: Modern synthetic reaction by Herbert O. House).
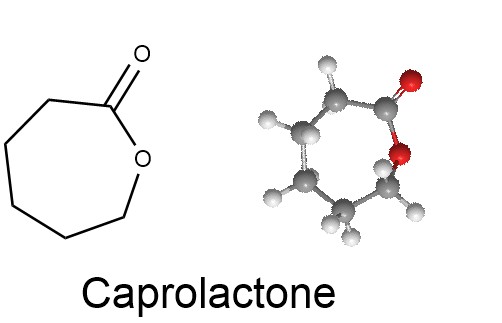
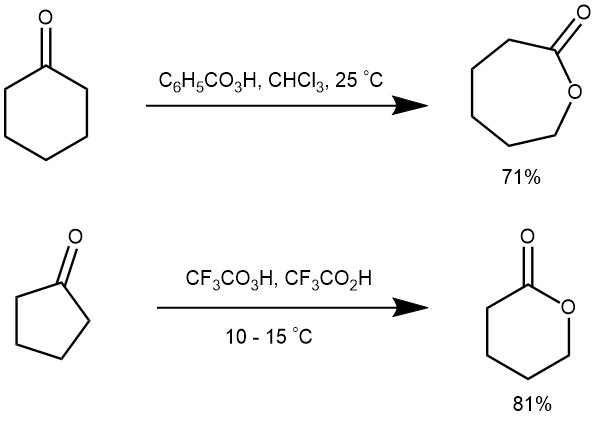
The oxidation of cyclic ketones with peracids serves as a useful route for lactones (cyclic ester).
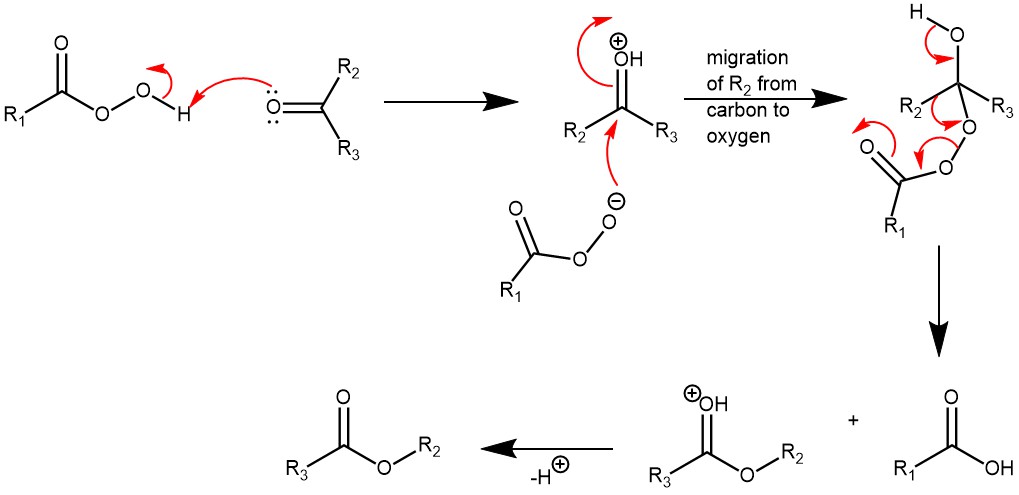
MECHANISM: The reaction is catalyzed by acid and the rate of oxidation is accelerated by electron-donating groups in the ketone and by electron-withdrawing groups in the peracids. The reaction also occurs with the retention of configuration.
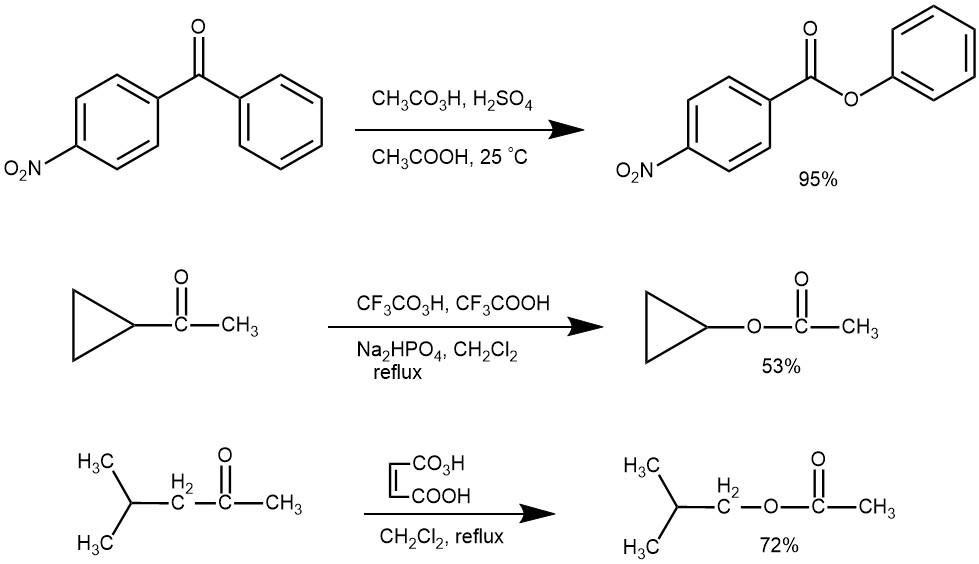
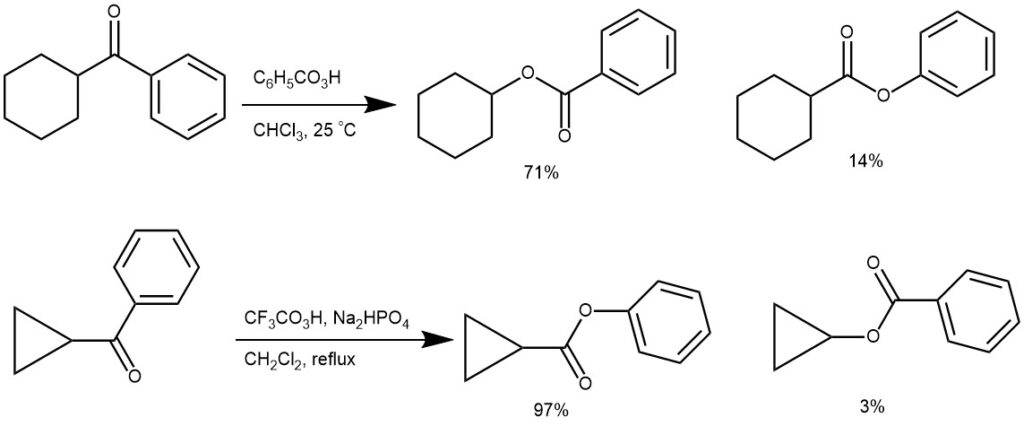
MIGRATORY APTITUTE: There is always a possibility of two isomeric esters for an unsymmetrical ketone. As can be seen from the examples below and also a study of a series of alkyl aryl ketones and from other studies, the relative ease of migration (migratory aptitude) of various groups in the Baeyer-Villiger oxidation reaction is: t-alkyl > cyclohexyl ~ sec-alkyl ~ benzyl ~ phenyl > primary > cyclopropyl > methyl.
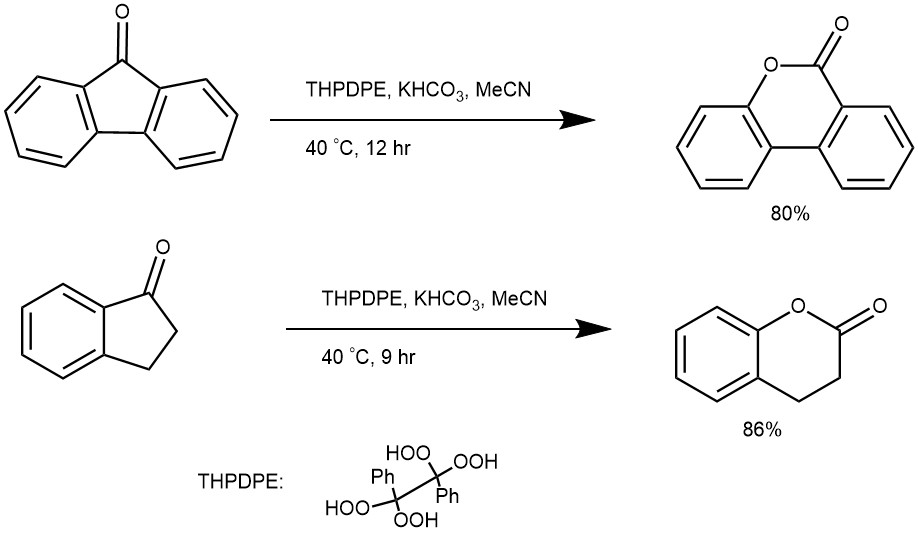
EXAMPLE 1: To a mixture of ketone (1 mmol) in CH3CN (4 mL), 1,1,2,2-tetrahydroperoxy-1,2-diphenylethane (THPDPE 1 mmol), and KHCO3 (1 mmol) was added and the solution was stirred at room temperature. After the completion of the reaction, as monitored by TLC, Na2SO3 (3 M, 1mL) and saturated NaCl (5 mL) were added to the mixture, and the corresponding products were extracted with CHCl3 (3 × 5 mL) (K. Khosravi, S. Naserifar / Tetrahedron 2018, 74, 6584-6592)
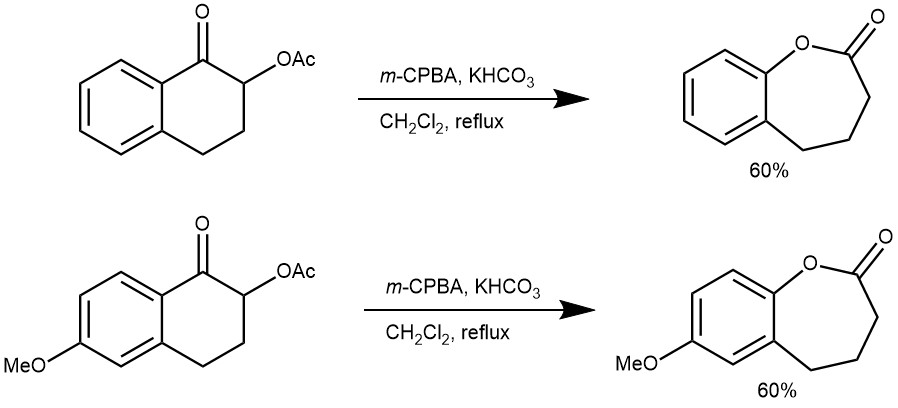
EXAMPLE 2: A solution of a-acetoxy ketones (50 mg, 0.28 mmol) and KHCO3 (35 mg, 0.35 mmol) in 10 mL CH 2 Cl2 were stirred, and commercial grade m-CPBA (80% activity, 61 mg, 0.35 mmol) was added to this mixture. The reaction mixture was stirred under reflux (REF: A.S. Demir, A. Aybey / Tetrahedron 2008, 64, 11256–11261)
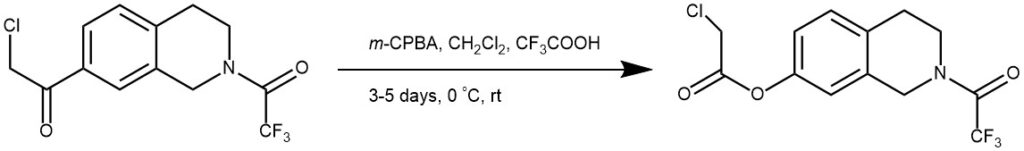
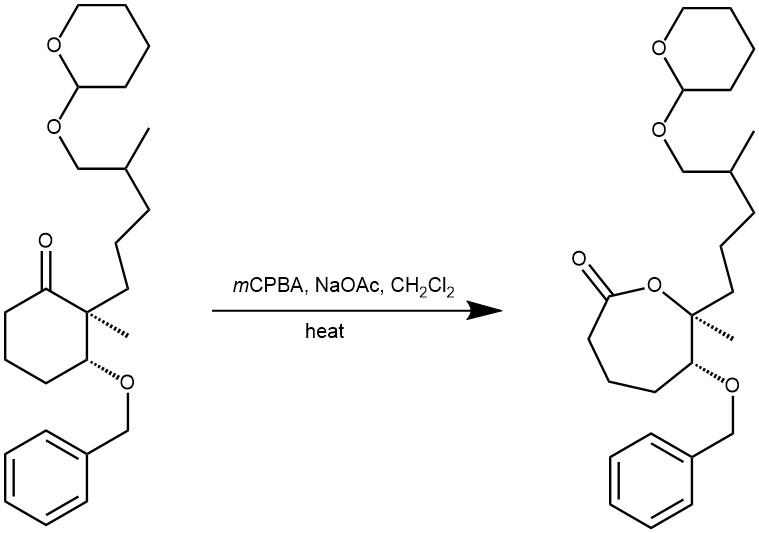
EXAMPLE 3: Compound (8.4 g, 27 mmol) was dissolved in anhydrous CH2Cl2 (58 ml) and 3-chloroperoxybenzoic acid (12.3 g) was added in one portion. The suspension was cooled to 0 °C, and trifluoroacetic acid (2.0 ml) was added dropwise over 5–10 min. The reaction flask was protected from light, and the mixture was stirred for 3–5 days at room temperature, poured onto water (100 ml), and neutralized with ammonium hydroxide solution. The layers were separated, and the aqueous layer was extracted with CH2Cl2 (60 ml). After purification on a silica gel column (n-hexane/EtOAc = 2:1) the title compound was obtained as a yellow oil (REF: M.-K. Hu et al. / Bioorg. Med. Chem. 2008, 16, 1957–1965).