Manganese dioxide (MnO2) is an oxidizing reagent for the selective oxidation of primary and secondary alcohols to their corresponding aldehydes and ketones. This reaction is widely used in organic synthesis and can be applied to a variety of alcohol substrates, including allylic, propargylic, benzylic, and heterocyclic alcohols, as well as saturated alcohols and 1,2-diols. The specific solvent used in the reaction depends on the type of alcohol being oxidized. For example, petroleum ether, hexane, acetone, and chloroform are commonly used solvents for allylic alcohols, while dichloromethane is preferred for propargylic alcohols and 1,2-diols.
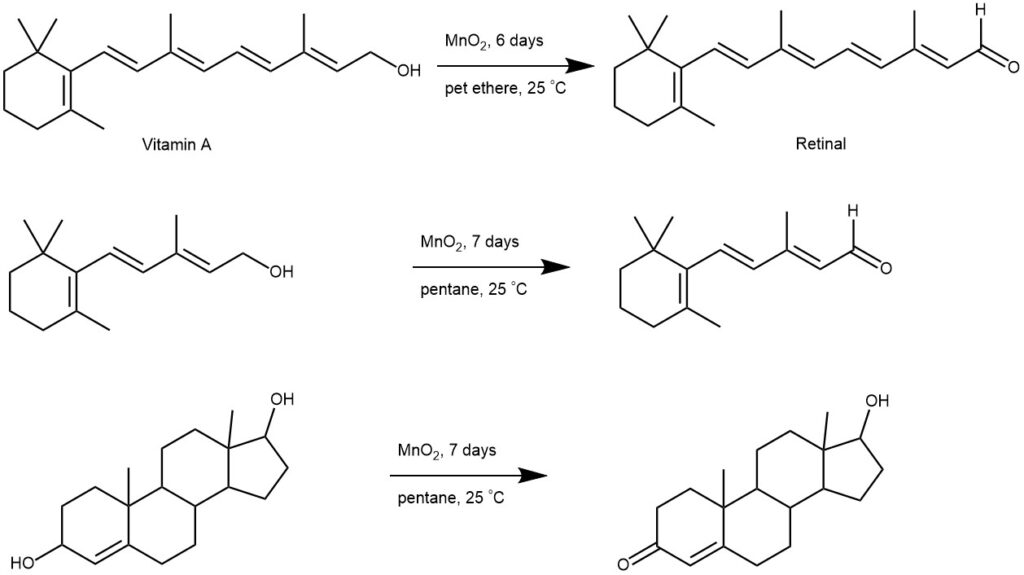
Manganese dioxide provides a more milder and selective method for the oxidation of allylic and benzylic alcohols to the corresponding aldehyde or ketone, at the expense of a relatively large excess of the oxidant. (Ref: Biochem. J. 1948, 42, 516; J. Am. Chem., Soc. 1951, 73, 719; J. Am. Chem. Soc., 1953, 75, 5930, 5932)
Manganese dioxide (MnO2) is a common form of Mn(IV). There are several ways of preparing it, however, one of the common methods involves the precipitation of MnO2 from a warm aqueous solution of MnSO4 and KMnO4. The precipitated reagent is then activated by heating to 100 – 200 °C or higher for several hours.
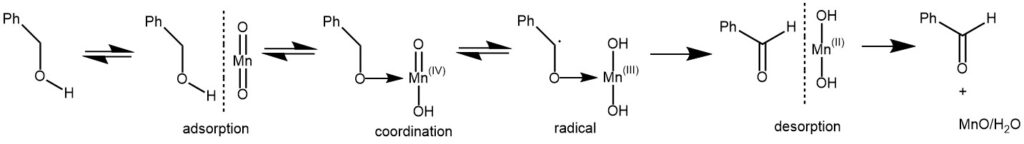
The mechanism involves the adsorption of the alcohol on manganese dioxide, followed by the formation of a coordination complex. Coordination in this manner allows electron transfer, which generates a radical in a process that is accompanied by the reduction of Mn(IV) to Mn(III). A second electron transfer generates the carbonyl product adsorbed on Mn(OH)2. The product is desorbed, with a loss of water, to complete the oxidation (J. Org. Chem. 1969, 34, 3289)
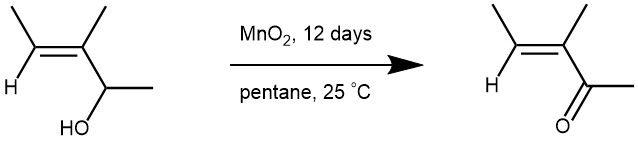
There is no significant difference in the rate of oxidation of cis – and trans-isomeric alkenes, and there appears to be little or no cis/trans isomerization during the oxidation (Ref: ibid., 1958, 80, 2428).
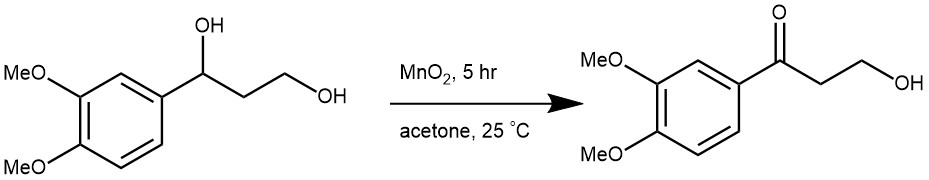
The secondary benzylic alcohol is oxidized selectively and relatively faster than a primary saturated alcohol.
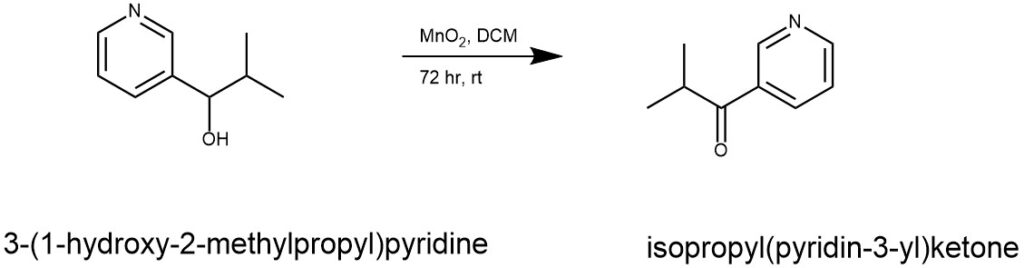
EXAMPLE 1: The starting alcohol (410 mg, 2.71 mmol) was dissolved in dichloromethane (10 mL). To this, manganese dioxide (3.08 g) was added at room temperature and the mixture was stirred at the same temperature for 72 hours. The reaction mixture was filtered through Celite and the mother liquid was concentrated. The residue was purified by silica gel column chromatography (hexane/ethyl acetate = 1/1) to give isopropyl(pyridin-3-yl)ketone (330 mg, yield: 82%). 1H-NMR (300 MHz, CDCl3, δ): 1.24 (s, 3H), 1.26 (s, 3H), 3.53 (sept, J = 6.9 Hz, 1H), 7.43 (ddd, J = 0.7, 4.8, 8.1 Hz, 1H), 8.23 (ddd, J = 1.8,2.2, 8.1 Hz, 1H), 8.78 (dd, J = 1.8, 4.8 Hz, 1H), 9.17 (dd, J = 0.7, 2.2 Hz, 1H). (Ref: World Intellectual Property Organization, WO2010053182 A1 2010-05-14)
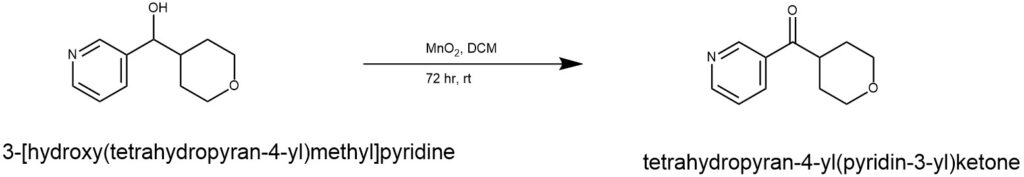
EXAMPLE 2: Manganese dioxide (6.38 g) was added to the alcohol (850 mg, 4.40 mmol) and dichloromethane (26 mL), and the mixture was stirred at room temperature. Then, purification by silica gel column chromatography (chloroform/methanol = 19/1) was performed to give tetrahydropyran-4-yl(pyridin-3-yl)ketone (480 mg, yield: 57%). ((Ref: World Intellectual Property Organization, WO2010053182 A1 2010-05-14)
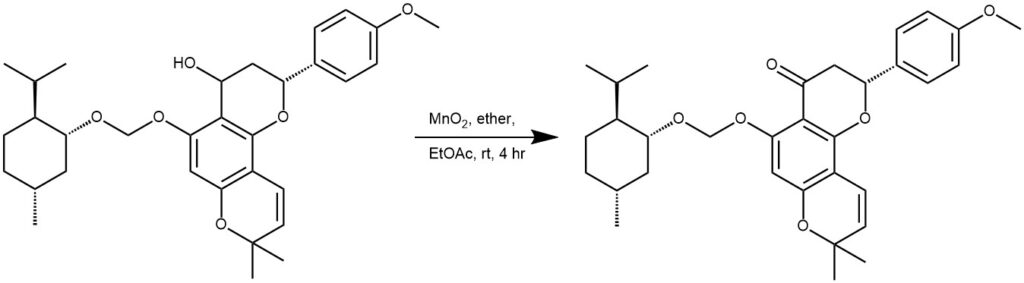
EXAMPLE 3: MnO2 (0.033 g, 0.38 mmol) was added to a solution of the SM (0.17 mmol) in 10 mL of ether and ethyl acetate (10 mL) at room temperature and stirred for 2 hours. An additional portion of MnO2 (0.074 g, 0.84 mmol) was added to the suspension and stirred for 2 more hours. The mixture was then filtered through celite and purified by column chromatography.
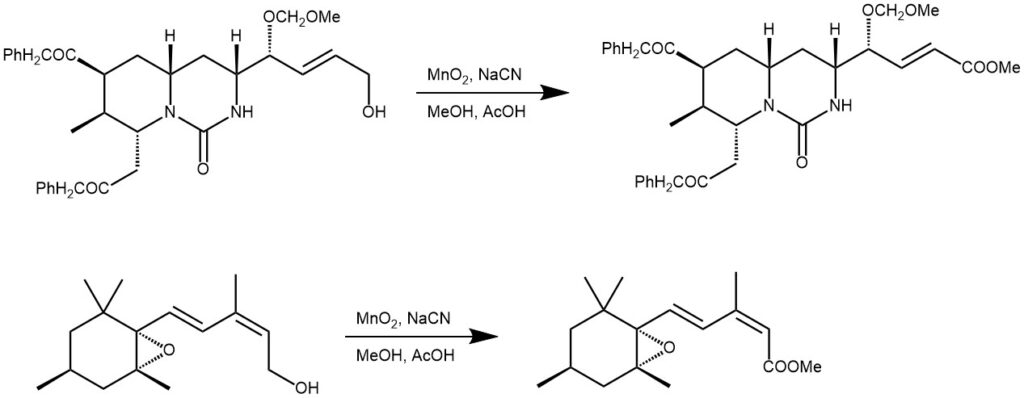
The allylic /benzylic alcohols can also react with MnO2in presence of NaCN and methanol to form methyl esters. It is also called as Corey-Gilman-Ganem reaction.