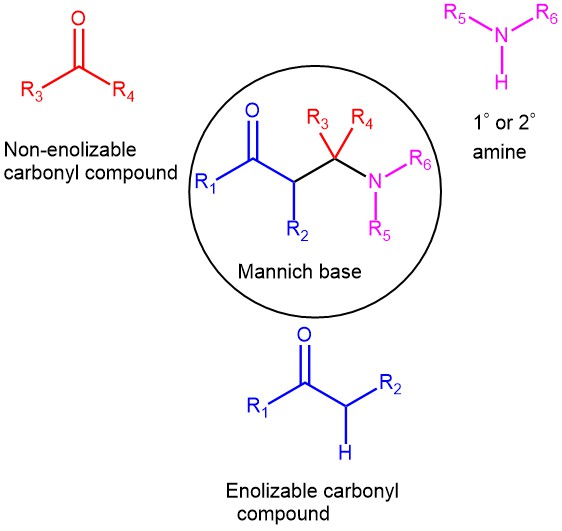
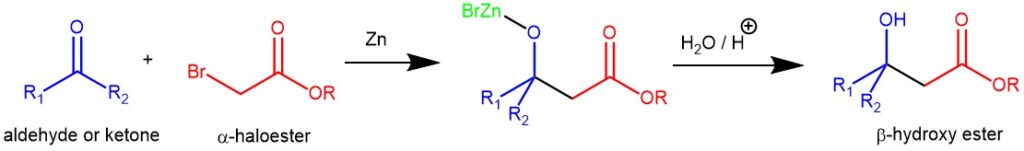
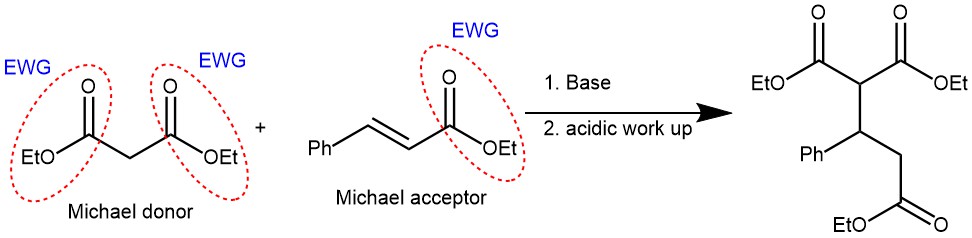
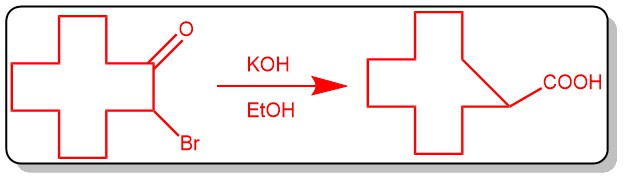
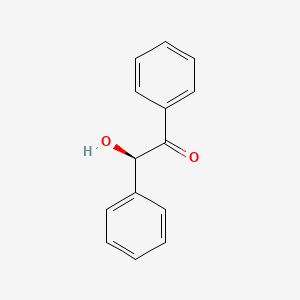
MANNICH REACTION
The condensation of a CH-activated compound (usually an aldehyde or ketone) with a primary or secondary amine (or ammonia) and a non-enolizable aldehyde (or ketone) to afford β-amino ketone is known as the Mannich reaction. In this transformation, three components, a ketone, an aldehyde, and an amine, react to form a β-amino ketone. The product …